Does the Carbon-Sulfur triple bond exist? There’s probably little doubt it does in the CS molecule. But now Schreiner and Mloston have offered up the H-C≡S-OH species as a possibility.1 Obtained by flash photolysis of 1, giving 2, and upon irradiation at 254 nm, H-C≡S-OH 3 is the observed species and not the expected carbene HO-C-SH 4. 3 is confirmed by excellent agreement between the observed and computationally predicted IR spectra.

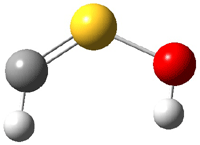
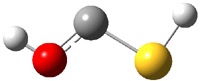
The CCSD(T)/cc-pVTZ structures of 3 and 4 are shown in Figure 1. It is interesting that the carbene is not observed, even though it is 26.6 kcal mol-1 more stable than 3.
|
3 |
4 |
Figure 1. CCSD(T)/cc-PVTZ optimized structures of 3 and 4.1
So is there a triple bond? The short C-S distance (1.547 Å) is very similar to that in CS (1.545 Å). NBO analysis indicates a triple bond. But the MOs indicate significant lone pair build-up on both C and S, consistent with the strongly non-linear angles about these two atoms. The authors conclude that 3 is a “structure with a rather strong CS double bond or a weak triple bond”.
References
(1) Schreiner, P. R.; Reisenauer, H. P.; Romanski, J.; Mloston, G., "A Formal Carbon-Sulfur Triple Bond: H-C≡S-O-H," Angew. Chem. Int. Ed., 2009, 48, 8133-8136, DOI: 10.1002/anie.200903969

The Nature of the CS triple bond « Henry Rzepa responded on 01 Dec 2009 at 6:18 am #
[…] Steve Bachrach has just blogged on a recent article (DOI: 10.1002/anie.200903969) claiming the isolation of a compound with a C≡S triple bond; A compound with a CS triple bond […]
Henry Rzepa responded on 01 Dec 2009 at 6:27 am #
A recurring theme in both this blog and my own is what the nature of bonding actually is. So I found this post irresistible, especially when I read that Schreiner and co in their comprehensive analysis of this little molecule had not actually applied the ELF technique. It does not take long to do and the results are posted here. A rather different take on the nature of the CS interaction emerges. Rather than being a weak triple bond, or a strong double one, the bond is seen in quite a different light! Whether ELF represents reality any more than NBO bond orders, or lengths, remains to be established of course.
Henry Rzepa responded on 30 Dec 2009 at 4:31 pm #
The issue of whether a CS bond can sustain triple character goes back to earlier analogues to the system discussed in this post, namely F3C-C≡SF3 and F3S-C≡SF3. For other reasons, I found F3S-C≡SF3 worthy of a calculation (it has a nice symmetry, but only as a pure carbene. The symmetry is destroyed if a triple bond is invoked). The results of an ELF analysis, I have to say, quite surprised me, and can be seen here. Which, I think, only goes to show that valency continues to hold surprises!