Medvedev, et al. have examined the cyclization step in the formation of Spinosyn A, which is catalyzed by the putative Diels-Alderase enzyme SpnF.1 This work follows on the computational study done by Houk, Singleton and co-workers,2 which I have discussed in this post (Dynamics in a reaction where a [6+4] and [4+2] cycloadditons compete). In fact, I recommend that you read the previous post before continuing on with this one. In summary, Houk, et al. found that a single transition state connects reactant 1 to both 2 and 3. The experimental product with the enzyme SpnF is 3. In the absence of enzyme, Houk, et al. suggest that reactions will cross the bispericyclic transition state TS-BPC (TS1 in the previous post) leading primarily to 2, which then undergoes a Cope rearrangement to get to product 3. Some molecules will follow pathways that go directly to 3.
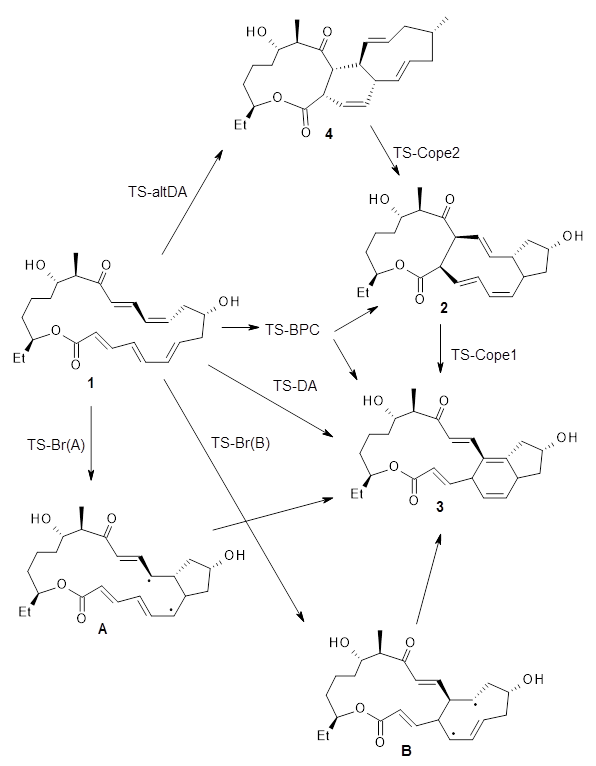
The PCM(water)/M06-2x/6-31+G(d) study by Medvedev, et al. first identifies 560 conformations of 3. Next, they identified 384 TSs lying within 30 kcal mol-1 from the lowest TS. These can be classified as either TS-DA (leading directly to 3) or TS-BPC (which may lead to 2 by steepest descent, but can bifurcate towards 3). They opt to utilize the Atoms-in-Molecules theory to identify bond critical points to categorize these TS, and find that 144 are TS-BPC and 240 are TS-DA. (The transition state found by Houk, et al. is the second lowest energy TS found in this study, 0.29 kcal mol-1 higher in energy that the lowest TS and also of TS-BPC type.)
They also examined two alternative routes. First, they propose a path that first takes 1 to 4 via an alternative Diels-Alder reaction, and a second Cope rearrangement (TS-Cope2) takes this to 2, which can then convert to 3 via TS-Cope1. The other route involves a biradical pathway to either A or B. These alternatives prove to be non-competitive, with transition state energies significantly higher than either TS-DA or TS-BPC.
Returning to the set of TS-DA and TS-BPC transition states, while the former are more numerous, the latter are lower in energy. In summary, this study further complicates the complex situation presented by Houk, et. al. In the absence of catalyst, 1 can undergo either a Diels-Alder reaction to 3, or pass through a bispericyclic transition state that can lead to 3, but principally to 2 and then undergo a Cope rearrangement to get to 3. The question that ends my previous post on this subject — “ just what role does the enzyme SpnF play?” — remains to be answered.
References
1) Medvedev, M. G.; Zeifman, A. A.; Novikov, F. N.; Bushmarinov, I. S.; Stroganov, O. V.; Titov, I. Y.; Chilov, G. G.; Svitanko, I. V., "Quantifying Possible Routes for SpnF-Catalyzed Formal Diels–Alder Cycloaddition." J. Am. Chem. Soc. 2017, 139, 3942-3945, DOI: 10.1021/jacs.6b13243.
2) Patel, A.; Chen, Z.; Yang, Z.; Gutiérrez, O.; Liu, H.-w.; Houk, K. N.; Singleton, D. A., "Dynamically Complex [6+4] and [4+2] Cycloadditions in the Biosynthesis of Spinosyn A." J. Am. Chem. Soc. 2016, 138, 3631-3634, DOI: 10.1021/jacs.6b00017.
InChIs
1: InChI=1S/C24H34O5/c1-3-21-15-12-17-23(27)19(2)22(26)16-10-7-9-14-20(25)13-8-5-4-6-11-18-24(28)29-21/h4-11,16,18-21,23,25,27H,3,12-15,17H2,1-2H3/b6-4+,8-5+,9-7+,16-10+,18-11+/t19-,20+,21-,23-/m0/s1
InChIKey=JEKALMRMHDPSQK-ZTRRSECRSA-N
2: InChI=1S/C24H34O5/c1-3-19-8-6-10-22(26)15(2)23(27)20-12-11-17-14-18(25)13-16(17)7-4-5-9-21(20)24(28)29-19/h4-5,7,9,11-12,15-22,25-26H,3,6,8,10,13-14H2,1-2H3/b7-4-,9-5+,12-11+/t15-,16-,17-,18-,19+,20+,21-,22+/m1/s1
InChIKey=AVLPWIGYFVTVTB-PTACFXJJSA-N
3: InChI=1S/C24H34O5/c1-3-19-5-4-6-22(26)15(2)23(27)11-10-20-16(9-12-24(28)29-19)7-8-17-13-18(25)14-21(17)20/h7-12,15-22,25-26H,3-6,13-14H2,1-2H3/b11-10+,12-9+/t15-,16+,17-,18-,19+,20-,21-,22+/m1/s1
InChIKey=BINMOURRBYQUKD-MBPIVLONSA-N

Henry Rzepa responded on 12 Apr 2017 at 6:14 am #
Searching the highlighted article for the term entropy does not throw up any hits. At its most simple, surely the role of an enzyme acts upon this contribution to the free energies of activation? Aspects such as hydrogen bonding and dispersion are probably only next in the list. So its a bit odd that the relative entropies (ΔSrel) of the various diverse routes are not discussed in this article. Table 1 for example reports only E(rel) for 14 different possibilities. I presume this means the difference in total energy computed? Not even apparently a ΔH‡ (corrected for ZPE),never mind a ΔG‡.
To see how entropy can affect the kinetics of a Diels-Alder cycloaddition, see here.
Michael G. Medvedev responded on 03 Jul 2017 at 5:36 am #
Dear Prof. Rzepa,
I am sorry for answering so late; I have just found this highlight.
Indeed, enzyme likely acts upon the entropic contribution. Actually, enzyme can affect this reaction in many different ways so we have not said that we have studied the reaction in the enzyme; as we said, we have studied the reaction in water (implicitly), and the most valuable outputs of our work (in my opinion) are: (1) this reaction has many low-lying possible transition states, all of which should be considered when studying it in the enzyme and (2) the structures of these transition states available in SI.
As stated in the table’s title, the given energies are thermally corrected, i.e. these are actually ΔΔG‡ (as defined in Fig. 1 of DOI:10.1016/j.mencom.2017.05.002). It is our omission that we have improperly named the column. Free energy values were also used in all plots.
Best regards, Michael G. Medvedev