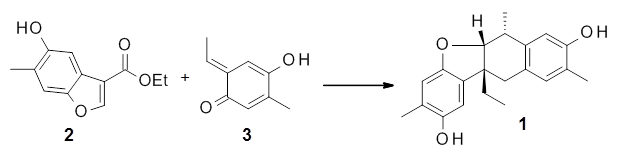
Xu, Liu, Xu, Gao, and Zhao report a very efficient synthesis of paeoveitol 1 by the [4+2]-cycloaddition of paeveitol D 2 with the o-quinone methide 3.1 What is interesting here is the selectivity of this reaction. In principle the cyloadditon can give four products (2 different regioisomeric additions along with endo/exo selectivity) and it could also proceed via a Michael addition.
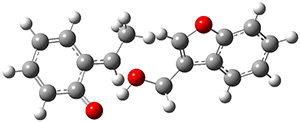
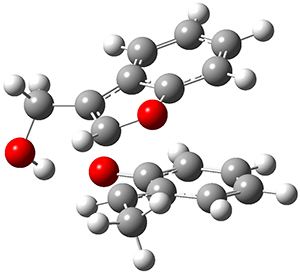
They performed PCM(CH2Cl2)/M06-2x/6-311+G(d,p) computations on the reaction of 2 with 3 and located two different transition states for the Michael addition and the four cycloaddition transition states. The lowest energy Michael and cycloaddition transition states are shown in Figure 1. The barrier for the cycloaddition is 17.6 kcal mol-1, 2.5 kcal mol-1 below that of the Michael addition. The barriers for the other cycloaddition paths are at more than 10 kcal mol-1 above the one shown. This cycloaddition TS is favored by a strong intermolecular hydrogen bond and by π-π-stacking. In agreement with experiment, it is the transition state that leads to the observed product.
|
Michael TS |
[4+2] TS |
Figure 1. Optimized geometries of the lowest energy TSs for the Michael and [4+2]cycloaddtion routes. Barrier heights (kcal mol-1) are listed in parenthesis.
References
(1) Xu, L.; Liu, F.; Xu, L.-W.; Gao, Z.; Zhao, Y.-M. "A Total Synthesis of Paeoveitol," Org. Lett. 2016, ASAP, DOI: 10.1021/acs.orglett.6b01736.
paeoveitol 1: InChI=1S/C21H24O3/c1-5-21-10-14-6-11(2)17(22)8-15(14)13(4)20(21)24-19-7-12(3)18(23)9-16(19)21/h6-9,13,20,22-23H,5,10H2,1-4H3/t13-,20-,21-/m1/s1
InChIKey=LCLFTLPUJXVULB-OBVPDXSSSA-N
paeveitol D 2: InChI=1S/C9H10O2/c1-3-7-5-8(10)6(2)4-9(7)11/h3-5,10H,1-2H3/b7-3+
InChIKey=KWDDAFOCZGDLEG-XVNBXDOJSA-N
3: InChI=1S/C9H10O2/c1-3-7-5-8(10)6(2)4-9(7)11/h3-5,10H,1-2H3/b7-3+
InChIKey=KWDDAFOCZGDLEG-XVNBXDOJSA-N