Bond-stretch isomerism refers to isomers that differ simply in their bond lengths. Seppelt and coworkers suggests that the hexafluorobenzne radical cation C6F6.+ exhibits bond-stretch isomerism.1
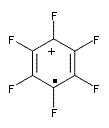
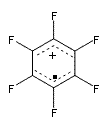
The oxidized C6F6 with O2+SbF11– and obtained C6F6.+Sb2F11– as a crystalline solid. X-ray diffraction identified 2 structures. B3LYP/TZPP computations confirmed the identity of two isomers, a “quinoid” form 1 and a “bisallyl” form 2, shown in Figure 1. The two structures are nearly degenerate, with 1 predicted to be 0.09 kcal mol-1 more stable than 2. The computed two unique C-C bond lengths are 1.371 and 1.427 Å in 1 and 1.449 and 1.389 Å in 2, and these distance agree well with the X-ray experimental values.
|
|
|
|
1 – quinoid |
2 – bisallyl |
Figure 1. UB3LYP/6-311+G(d) optimized structures of 1 and 2. Note once again the article and supporting materials lacked the full description of these structures!)
The potential energy surface in the neighborhood of these two isomers is like that of a sombrero. The two isomers lie in the circular trough and movement around this trough is nearly flat. The peak of the sombrero is the D6h structure, which is a transition state interconverting 1 and 2, with a barrier of 3 kcal mol-1.
1 and 2 are clear examples of bond-stretch isomerism, though it is likely that the complexation with the counter ion is what freezes out the rapid interconversion of the two.
References

Henry Rzepa responded on 09 Sep 2009 at 12:43 am #
Bond stretch isomerism is also found in neutral annulenes. With aromatic annulenes such as benzene, the so-called Kekulé mode (b2u) stretches three bonds and contracts the other three. This mode has a positive force constant (albeit depressed from its anticipated value, but that is a different story, as Sason Shaik has shown. See also DOI: 0.1039/B911817A). But for other (anti-aromatic) annulenes, this Kekulé mode can have a negative force constant (i.e. a transition state, as Steve notes in the blog). One example would be [16]annulene, which has two isomers, differing in the C-C bond lengths. Indeed, beyond around the [26]annulene stage, it is thought that ALL higher annulenes will exhibit such isomerism. The difference here is of course that the two bond stretch isomers are identical, and that their interconversion is narcissistic. The effect by the way, HAS been established crystallographically for an anti-aromatic [28] hexaphyrin (see DOI: 10.1021/ol703129z!
For the example in Steve’s blog, I assume that the isomerism could also be simply described as a Jahn-Teller distortion, since removing one electron from hexaflurobenzene must be from a degenerate π-MO, and hence there are two ways of performing this removal, hence two isomers. One might predict by the same criterion that the radical anion of hexafluorobenzene might show a similar effect. Anyone up for this calculation? The question there would be whether the Kekulé mode for this radical anion has a positive or a negative force constant.
And finally, one might note that the first excited state of benzene may also show bond stretch isomerism? Any comments on that one?