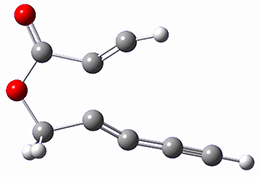
Cramer, Hoye, Kuwata and coworkers have examined the intramolecular cyclization of an alkyne with a diyne.1 Their model system is 1, which can cyclize through a concerted transition state TSC togive the benzyne product 2, or it can proceed through a stepwise pathway, first going through TS1 to form the intermediate INT¸ before traversing through a second transition state TS2 and on to product 2. Using both computations and experiments, they examined a series of compounds with
differing substituents at the ends of the two yne moieties.
The experiments show almost the exact same rate of reaction regardless of the terminal substituents. This includes the parent case where the terminal substituents are hydrogens and also the case where the terminal substituents (which end up on adjacent centers on the benzyne ring) are bulky TMS groups. And though there is no real rate effect due to changes in solvent or the presence of light or triplet oxygen, which suggest a concerted reaction, these substituent effects argue for a step wise reaction.
SMD(o-dichlorobenzene)/B3LYP-D3BJ/6-311+G-(d,p)//M06-2X/6-311+G(d,p)
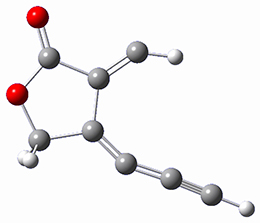
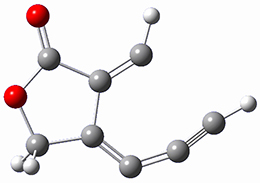
computations help explain these observations. Shown in Figure 1 are the optimized geometries and relative energies of the critical points on the reaction surface for the conversion of 1 into 2, and these results are similar with the other substituents as well.
|
1 |
2 |
|
TSC |
|
|
TS1 |
INT |
|
TS2 |
|
Figure 1. SMD(o-dichlorobenzene)/B3LYP-D3BJ/6-311+G-(d,p)//M06-2X/6-311+G(d,p) optimized geometries and relative energies (kcal mol-1).
The first thing to note is that the concerted TSC is higher in energy than the stepwise TS1. The wavefunction for TSC unstable towards moving from a restricted to unrestricted formalism. Reoptimization of some of these concerted TSs actually led to the stepwise TS.
The next item of note is that TS2 for this case is actually lower in energy than the intermediate (this is a true TS on the energy surface, but when zero-point energy and other thermal corrections are included, it becomes lower in energy than INT). For some of the cases the second TS lies above the intermediate, but typically by a small amount.
Therefore, the mechanism of this reaction is stepwise, but the second step might have such a small barrier (or even no barrier) that one might consider this to be concerted, though highly asymmetric and really bearing little resemblance to more traditional concerted pericyclic reactions.
The authors obliquely hinted at some potential interesting dynamics. I suspect that molecular dynamics calculations will show no effect of that second TS, and one might observe some interesting dynamics, which could be seen in kinetic isotope experiments.
References
(1) Marell, D. J.; Furan, L. R.; Woods, B. P.; Lei, X.; Bendelsmith, A. J.; Cramer, C. J.; Hoye, T. R.; Kuwata, K. T. "Mechanism of the Intramolecular Hexadehydro-Diels–Alder Reaction," J. Org. Chem. 2015 ASAP, DOI: 10.1021/acs.joc.5b01356.
InChIs
1: InChI=1S/C8H4O2/c1-3-5-6-7-10-8(9)4-2/h1-2H,7H2
InChIKey=MGXDIFXPYGGQLF-UHFFFAOYSA-N
2: InChI=1S/C8H4O2/c9-8-7-4-2-1-3-6(7)5-10-8/h2,4H,5H2
InChIKey=MYFORDRJCVOBTH-UHFFFAOYSA-N

George Dionne responded on 12 Oct 2015 at 1:51 am #
Thank you for sharing. Hope to hear more from you.
Henry Rzepa responded on 26 Oct 2015 at 8:46 am #
The method SMD(o-dichlorobenzene)/B3LYP-D3BJ/6-311+G-(d,p)//M06-2X/6-311+G(d,p) caught my eye, since it separates the solvation Hamiltonian from the geometry Hamiltonian (the // in the method).
Using this separated method, TSC is described as having a wavefunction unstable to open shell configurations obtained using the unrestricted formalism. I asked myself whether this instability would still be true if a calculation combining continuum solvation and geometry optimisation were tried. Under these conditions (M06-2X/6-311+G(d,p)/SCRF=o-dichlorobenzene), the wavefunction for the re-optimized transition state geometry turns out to be stable (doi:8p3), which goes to show that such triplet instabilities might in fact be removed by solvation stabilisation. The entire surface is concerted, as revealed by an intrinsic reaction coordinate performed along its entire course with continuum solvation applied (below).
TS1, Int and TS2 are all UHF unstable as biradicals and this continues to be true with solvation applied. The path TS1 → Int also shows up nicely in the IRC (doi: 8p4, 8p5), but there is an incompleteness in the description above, since there is apparently direct no IRC path from Int → TS2!
Instead Int isomerizes to Int1, involving inversion at the C=C-H centre via a linear transition state arrangement (doi: 8p6, 8p7) and it is Int1 that now shows a continuous IRC (doi: 8p8, 8p9) from it via TS2 to 2.
This shows how the concerted (TSC) and stepwise (TS1, TS2 and TS-Int) pathways can all co-exist in the potential energy surface, and reinforces the suggestion that this system will show some interesting dynamics (assuming that solutions to the technical problems of ensuring that the spin-symmetry breaking is maintained in a continuous manner along the dynamic trajectories can be found). For example, the Int1 → TS2 → 2 trajectory corresponding to the IRC shown below is in part biradical and in part closed shell and this does have to be handled in a continuous manner (see eg http://www.ch.imperial.ac.uk/rzepa/blog/?p=12115 for an example).