Yang, Ganz, Chen, Wang, and Schleyer have published a very interesting and comprehensive review of planar hypercoordinate compounds, with a particular emphasis on planar tetracoordinate carbon compounds.1 A good deal of this review covers computational results.
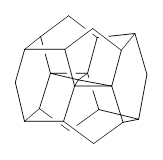
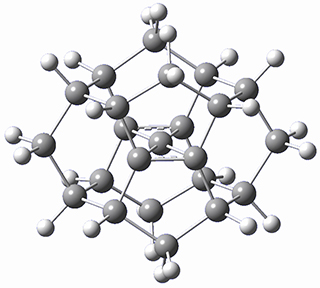
There are two major motifs for constructing planar tetracoordinate carbon compounds. The first involves some structural constraints that hold (or force) the carbon into planarity. A fascinating example is 1 computed by Rasmussen and Radom in 1999.2 This molecule taxed their computational resources, and as was probably quite typical for that time, there is no supplementary materials. But since this compound has high symmetry (D2h) I reoptimized its structure at ω-B97X-D/6-311+G(d) and computed its frequencies in just a few hours. This structure is shown in Figure 1. However, it should be noted that at this computational level, 1 possesses a single imaginary frequency corresponding to breaking the planarity of the central carbon atom. Rasmussen and Radom computed the structure of 1 at MP2/6-31G(d) with numerical frequencies all being positive. They also note that the B3LYP/6-311+G(3df,2p) structure also has a single imaginary frequency.
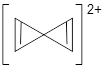
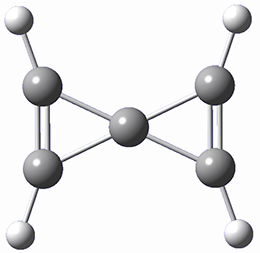
A second approach toward planar tetracoordinate carbon compounds is electronic: having π-acceptor ligands to stabilize the p-lone pair on carbon and σ-donating ligands to help supply sufficient electrons to cover the four bonds. Perhaps the premier simple example of this is the dication 2¸ whose ω-B97X-D/6-311+G(d,p) structure is also shown in Figure 1.
The review covers heteroatom planar hypercoordinate species as well. It also provides brief coverage of some synthesized examples.
|
|
|
|
1 |
2 |
Figure 1. Optimized structures of 1 and 2.
References
(1) Yang, L.-M.; Ganz, E.; Chen, Z.; Wang, Z.-X.; Schleyer, P. v. R. "Four Decades of the Chemistry of Planar Hypercoordinate Compounds," Angew. Chem. Int. Ed. 2015, 54, 9468-9501, DOI: 10.1002/anie.201410407.
(2) Rasmussen, D. R.; Radom, L. "Planar-Tetracoordinate Carbon in a Neutral Saturated Hydrocarbon: Theoretical Design and Characterization," Angew. Chem. Int. Ed. 1999, 38, 2875-2878, DOI: 10.1002/(SICI)1521-3773(19991004)38:19<2875::AID-ANIE2875>3.0.CO;2-D.
InChIs
1: InChI=1S/C23H24/c1-7-11-3-15-9-2-10-17-5-13-8(1)14-6-18(10)22-16(9)4-12(7)20(14,22)23(22)19(11,13)21(15,17)23/h7-18H,1-6H2
InChIKey=LMDPKFRIIOUORN-UHFFFAOYSA-N
2: InChI=1S/C5H4/c1-2-5(1)3-4-5/h1-4H/q+2
InChIKey=UGGTXIMRHSZRSQ-UHFFFAOYSA-N

Henry Rzepa responded on 28 Aug 2015 at 7:07 am #
Compound 2 is certainly intriguing. Being planar, it has four electrons in two π-orbitals, and I wondered how its cyclic conjugation might be described?
1. One 4n (n=1) cyclic conjugated system, nominally antiaromatic
2. Two 4n+2 (n=0) cyclic cross-conjugated rings, each aromatic (aka cyclopropenium cation)
3. Coarctate Hückel aromatic?
The calculated 1H NMR shifts (doi: 66j) are around 10.4 ppm, which suggests they might be in the deshielding region of an aromatic ring current. The MOs indicate one delocalised π-orbital covering all six atoms, and the second less stable with a node at the central carbon. The two NBOs localise onto each of the C=C units. The four bonds to the central carbon are 1.48Å, which are nominally too long to indicate cyclic conjugation. But that central atom may be anomalous in this regard.
I think the ring currents of this species might reveal something interesting!
So, community, is this molecule aromatic, antiaromatic or other?
Henry Rzepa responded on 29 Aug 2015 at 2:16 am #
Further thoughts about molecule 2. As a dictation, the only prospect of detection in this form would be in the gas phase in eg a mass spectrometer. So it would be of interest to see if it can survive in the presence of counter-ions. One of the simplest realistic counter-ions to use is the chloride anion, as 2.2Cl(-), which then raises the (faint?) prospects of solid isolations. The question then is whether the chloride will remain ionic, or whether it will form covalent C-Cl bonds, and what would happen then. As a naked Cl(-) ion, a distribution that results in D2h symmetry results in a species with a C-Cl bond length of 3.01Å (doi: 66t). However, even with a continuum solvation field for water applied to stabilise the anion, this species has three small -ve force constants. I was able to locate a stable sandwich-like ion-triple with ionic chlorides as implied by an ionic C-Cl bond length of 3.19Å and C2h symmetry (doi: 66v) only if a further explicit water molecule was added to each chloride anion. The predicted 1H NMR shifts were around +10.0ppm, pretty much as I reported above.
I conclude from this that the di-cation can (computationally) form an ionic ion-triple which is a minimum in the PES, and so the prospects of isolation are perhaps not as faint as might otherwise be assumed.
PS Above, I am endeavouring to practice what in the UK (and elsewhere?) is referred to as RDM, or Research Data Management. One feature of such RDM is that data should be allocated a doi to render it FAIR (Findable, accessible, inter-operable and re-usable). There are two such dois embedded above. The other aspect of RDM is that it is now mandatory for research outputs funded by “the UK tax payer” (ie funding councils).
Alan Shusterman responded on 29 Aug 2015 at 2:15 pm #
Fascinating topic. Thanks for this summary.
Some comments about terminology: ‘planar tetrahedral’ combines two geometric labels in a contradictory way. How can C be both planar and tetrahedral? ‘Planar hypercoordinate’ avoids this dilemma, but it creates another: what evidence is there for hypercoordination? The displayed images in this post show 4 neighbors around the planar C. What am I missing?
Henry Rzepa responded on 31 Aug 2015 at 2:27 am #
Re: the semantics of planar tetrahedral or other, the term four-coordinate square planar seems appropriately neutral. My favourite hypercoordinated element is hydrogen, which appears capable of sustaining genuine octahedral six-fold hypercoordination. And boron can be up to 11-coordinate in CH12B11 (-) or B12H12(2-), and arguably since 11 ELF basins can be detected for one boron, it is clearly also hypervalent.
The introduces the semantic difficulty of the distinction between hypercoordination and hypervalency. Put another way, what does a valency imply about an electron count? 11-coordinate boron is of course bounded by 8 valence shell electrons. If you permit a valence to be defined by ~0.7 electrons (why not?), then the term hypervalent and hypercoordinate are synonymous. It is only if you attempt to define a valency by eg 2 electrons that you start having issues.
Steven Bachrach responded on 31 Aug 2015 at 7:45 am #
I was a bit sloppy in writing this post. I agree that planar tetrahedral is misleading if not downright contradictory, and that the better language is planar tetracoordinate. I have gone back and changed these throughout my post. Thanks, Alan, for pointing this out.
Henry Rzepa responded on 06 Sep 2015 at 4:54 am #
Intrigued by how closely known compounds might eg approach planar tetracoordinate carbon, I rattled of this search, although it really just teases rather than solves. With the recent introduction of a Python-based API for the CSD (and the hope that perhaps something similar might be released for COD) I think a proper solution should be possible. I would argue that such inspection should become standard practice whenever reporting unusual structures determined just by computation.