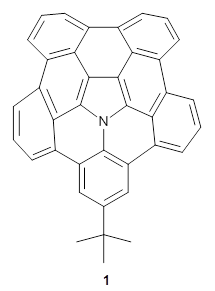
A heterosubstituted corranulene analogue has now been prepared. Ito, Tokimaru, and Nozaki report the synthesis of 1 and compare it with corranulene.1 The x-ray structure of 1 shows it to be a deeper bowl than corranulene, and the bond distances suggest the Kekule structure with a central pyrrole and five Clar-type phenyl rings.
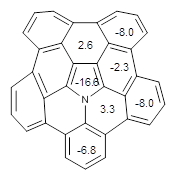
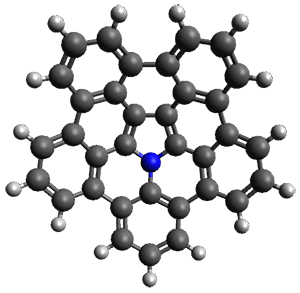
The B3LYP/6-311+G(2d,p) optimized structure of 2, then analogue of 1 missing the t-butyl group, is shown in Figure 1. Its geometry is very similar to that of 1 observed in the crystal structure. The NICS(0) values are shown in Scheme 1. These values support the notion of a central (aromatic) pyrrole surrounded by a periphery of five aromatic phenyl rings.
Scheme 1. NICS(0) values
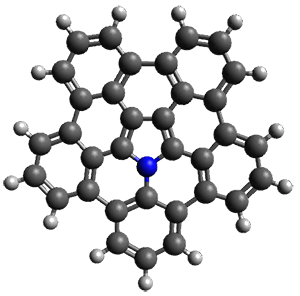
An interesting feature of bowl compounds is their inversion. The inversion barrier, through the planar TS shown in Figure 2, is computed to be 17.0 kcal mol-1 at B3LYP/6-311+G(2d,p). This is 6-7 kcal mol-1 larger than the inversion barrier of corranulene, which is not surprising given the additional phenyl groups about the periphery.
|
2 |
bowl inversion TS |
Figure 1. B3LYP/6-311+G(2d,p) optimized geometry of 2.
References
(1) Ito, S.; Tokimaru, Y.; Nozaki, K. "Benzene-Fused Azacorannulene Bearing an Internal Nitrogen Atom," Angew. Chem. Int. Ed. 2015, 54, 7256-7260, DOI: 10.1002/anie.201502599.
InChI
1: InChI=1S/C38H23N/c1-38(2,3)18-16-27-25-14-6-12-23-21-10-4-8-19-20-9-5-11-22-24-13-7-15-26-28(17-18)35(27)39-36(31(23)25)33(29(19)21)34(30(20)22)37(39)32(24)26/h4-17H,1-3H3
InChIKey= JJPREOOFFSVARV-UHFFFAOYSA-N
2: InChI=1S/C34H15N/c1-6-16-17-7-2-9-19-21-11-4-13-23-25-15-5-14-24-22-12-3-10-20-18(8-1)26(16)30-31(27(17)19)34(29(21)23)35(32(24)25)33(30)28(20)22/h1-15H
InChIKey= FFFUKUDWQMOFQQ-UHFFFAOYSA-N

Henry Rzepa responded on 27 Jul 2015 at 3:26 am #
A comment made in the article you discuss caught my eye: This phenomenon suggests that some self-aggregation of 3a occurs in the solution state. The association constant determined by using an indefinite noncooperative (isodesmic) model at 25°C was 5.0 ± 0.4.
An obvious property to start probing this point would be the MEP (molecular electrostatic potential), of interest to see how the nitrogen “lone pair” impinges and how the convex and concave surfaces differ.
(the calculation from which this MEP is derived is archived at DOI: 10.14469/ch/191394)
Perhaps one might conclude from this that the stacking alternates the position of the N atom, which would then make the convex/concave surfaces complementary. The reported crystal structure shows just such stacking (DOI: 10.5517/cc14hv4m).
I have included the crystal structure and calculation DOIs here, since I think its useful to show not only where the story is told (the journal article) but also where data relevant to the story is held (DataDOIs). If you are wondering how I got the DOI for the crystal structure, I used https://summary.ccdc.cam.ac.uk/structure-summary-form into which the publication DOI can be entered to retrieve the crystal DOI. The data itself can be directly downloaded from the search result page that appears, and you also get a JSmol 3D view similar to the one you can also get in the post above.
At the present moment, few publications cite BOTH the narrative AND the data DOIs, but perhaps if the habit spreads from blogs such as this one, we will all benefit from having simple and immediate access to the data from which scientific conclusions are based. Oh, and I should mention that a (FAIR = findable, accessible, interoperable and reusable) DataDOI should not just be to the supporting information associated with the original narrative; it should be fully enabled in its own right in an environment suited for data. A PDF file (which is where all journal supporting information currently appears) is not FAIR. If you are not sure what an appropriate environment is, go visit either of the two dataDOIs I cite above to find out.
George Dionne responded on 30 Jul 2015 at 7:13 am #
Thanks for posting! The images really help too. Looking forward to your updates.