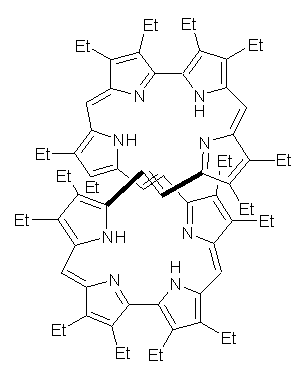
The octaphyrin 1 has been prepared and its crystal structure and electronic circular dichroism (ECD) spectra reported.1 The x-ray structure identified the compound as having the M,M helical structure. The optical rotation however could not be determined.
|
|
Rzepa now reports the computed ECD spectrum and optical activity of 1 and some related compounds.2 These computed spectra were obtained using TD0DFT with the B3LYP/6-31G(d) method with the CPCM treatment of the dichloromethane solvent. (The structure of 1 and other computed properties are available from the enhanced web table that Rzepa has deposited with the article (here). Once again this material seems to be available only to subscribers! My repeated discussions with ACS Pubs people that these “web objects” should be treated as data and not as copyrighted materials have fallen on deaf ears.) The computed ECD spectrum matches nicely with the experimental one, except that the signs at 570 and 620 nm are opposite. Rzepa suggests that either the compound is really of P,P configuration or the authors of experimental work have erroneously switched their assignments.
The computed value of [α]D of 1 is about -4000 °, with the negative sign in agreement with the sign for [α]D of M-hexahelicene. However, what is truly fantastic is the magnitude of the optical activity of the dication of 1 produced by loss of 2 electrons. This dication should be aromatic and it is predicted to have [α]1000 = -31597°!
References
(1) Werner, A.; Michels, M.; Zander, L.; Lex, J.; Vogel, E., ""Figure Eight" Cyclooctapyrroles: Enantiomeric Separation and Determination of the Absolute Configuration of a Binuclear Metal Complex," Angew. Chem. Int. Ed. 1999, 38, 3650-3653, DOI: 10.1002/(SICI)1521-3773(19991216)38:24<3650::AID-ANIE3650>3.0.CO;2-F
(2) Rzepa, H. S., "The Chiro-optical Properties of a Lemniscular Octaphyrin," Org. Lett. 2009, 11, 3088–3091DOI: 10.1021/ol901172g

Henry Rzepa responded on 02 Sep 2009 at 12:32 pm #
Steve notes that the “web objects” are once again only available to subscribers. Certainly, the material is NOT exclusively copyrighted by the ACS. I think its invisibility is simply an inexperienced editorial assistant not realising. I will contact the ACS, since I too care deeply that this material SHOULD be accessible to those who do not have access to the actual journal.
Watch this space
Henry Rzepa responded on 07 Sep 2009 at 9:47 am #
A small update to the above. Having checked, it seems that the “web objects” have not been correctly placed in the journal page for this particular article. They all download as ZIP files directly from the subscriber area, rather than existing in their own separate area. This is very probably due to a sub-editor who has never encountered these types of object before, and did not know what to do with them!
For the record (and Steve mentions this occasionally in his blog entries), I decided to try to collect in one place all the enhanced articles we have produced ourselves. The distinction might be a fine one. Thus would supporting information count? I argue that the data that in effect enhances the article is part of the primary publication, and not the supporting section. More importantly, this data is presented in a useful form to the reader, and not merely as TIFF images of scanned spectra or log files! Even more subtle, is the hope that the data will be made available not merely to the human reader, but to the remorseless robot, which will do things to it that we can only dream of. But I have already digressed to another topic!