The structure of water about a solute remains of critical importance towards understanding aqueous solvation. Microwave spectroscopy and computations are the best tools we have today to gain insight on this problem. This is nicely demonstrated in the Alonso study of the microsolvated structures of β-propiolactone 1.1 They employed chirped-pulse Fourier transform microwave (CP-FTMW) spectroscopy and MP2(fc)/6-311++G(d,p) computations to examine the structure involving 1-5 water molecules.
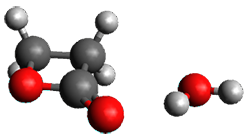
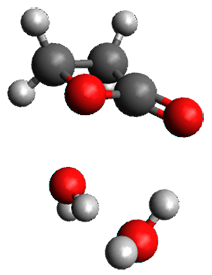
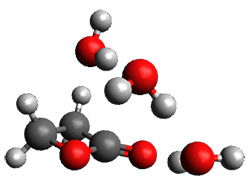
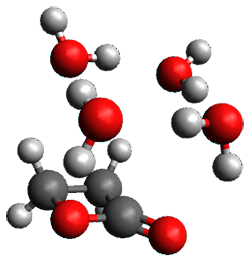
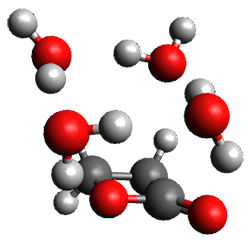
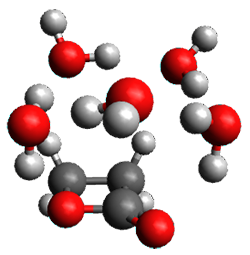
The computed structures of these microsolvated species are shown in Figure 1. The deviation of the computed and experimental structures (RMS in the atomic positions) is small, though increasing as the size of the cluster increases. The deviation is 0.014 Å for the 1. H2O cluster and 0.244 Å for the 1.(H2O)5 cluster. They identified two clusters with four water molecules; the lower energy structure, labeled as a, is only 0.2 kJ mol-1 more stable than structure b.
|
1.H2O |
1.(H2O)2 |
|
1.(H2O)3 |
|
|
1.(H2O)4 a |
1.(H2O)4 b |
|
1.(H2O)5 |
|
Figure 1. MP2(fc)/6-311++G(d,p) optimized geometries of the hydrates of 1.
Water rings are found in the clusters having four or five water molecules, while chains are identified in the smaller clusters. One might imagine water cages appearing with even more water molecules in the microsolvated structures.
References
(1) Pérez, C.; Neill, J. L.; Muckle, M. T.; Zaleski, D. P.; Peña, I.; Lopez, J. C.; Alonso, J. L.; Pate, B. H. Angew. Chem. Int. Ed. 2015, 54, 979-982, DOI: 10.1002/anie.201409057.
InChIs
1: InChI=1S/C3H4O2/c4-3-1-2-5-3/h1-2H2
InChIKey=VEZXCJBBBCKRPI-UHFFFAOYSA-N

Henry Rzepa responded on 28 Feb 2015 at 8:07 am #
I note a rather different type of experiment which casts light on the structures of microsolvated species; is eg doi:10.1103/PhysRevLett.114.043401. This one poses the nicely simple question “How many water molecules does it take to ionise HCl?” (the answer is 5-6).
I have shown some possible structures for these species (see also doi:10.15200/winn.142410.09115) and the cluster where ionisation actually occurs is also a bicyclic ring, with the water molecules forming a salt-bridge, just as Steve shows above for a neutral molecule.