Houk’s theory of torquoselectivity is a great achievement of computational chemistry, as told in Chapter 4.6 of the second edition of my book. Houk, in a collaboration with Krenske and Hsung, now report on an application of torquoselectivity in the formation of a cis-trans-cyclooctadienone intermediate.1
The proposed reaction is shown in Scheme 1, where the bicyclic compound undergoes a conrotatory ring opening in just one orientation to form the E,E-cyclooctadienone, which can then ring close to product.
Scheme 1.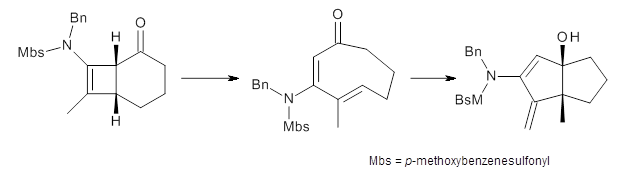
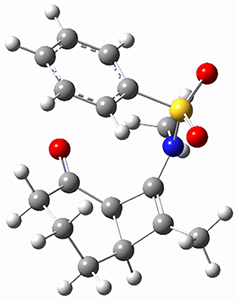
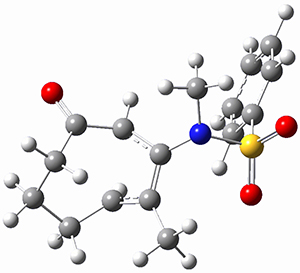
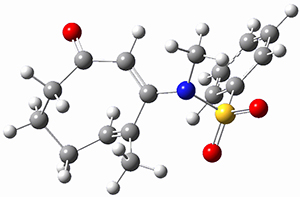
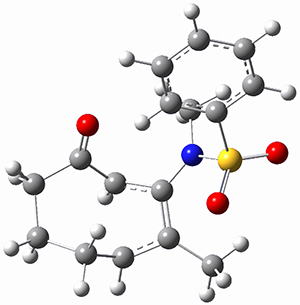
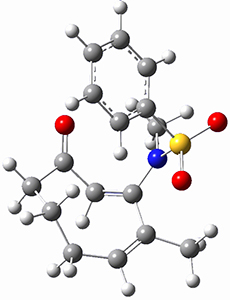
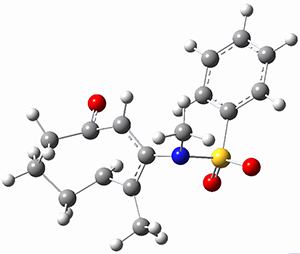
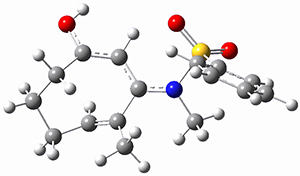
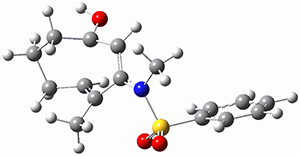
Houk ran M06-2x//6-311+G(d,p)//B3LYP/6-31G(d) computations on the model system 1, passing over the two torquodistinctive transition states TSEE and TSZZ, and on to produce the two cyclooctadienones 2EE and 2ZZ, respectively. As seen in Figure 1, the barrier through TSEE is favored by 9.8 kcal mol-1, and leads to the much more favorable cycloocatadienone 2EE.
|
1 |
|
|
TSEE |
2EE |
|
TSZZ |
2ZZ |
|
TS2 |
|
Figure 1. B3LYP/6-31G(d) optimized structures and relative free energies (kcal mol-1) at M06-2x//6-311+G(d,p)//B3LYP/6-31G(d).
Ring closure taking TSEE to product goes through TS2 (Figure 1), with a very high barrier, 47.5 kcal mol-1 above reactant, suggesting that this path is not likely to occur. Instead, they propose that 2EE is first protonated (2EEH+) and then cyclizes through TS2H+ (Figure 2). This barrier is only 6.2 kcal mol-1, some 44 kcal mol-1 lower than the neutral process through TS2.
|
2EEH+ |
TS2H+ |
Figure 2. B3LYP/6-31G(d) optimized structures
References
(1) Wang, X.-N.; Krenske, E. H.; Johnston, R. C.; Houk, K. N.; Hsung, R. P. "Torquoselective Ring Opening of Fused Cyclobutenamides: Evidence for a Cis,Trans-Cyclooctadienone Intermediate," J. Am. Chem. Soc. 2014, 136, 9802-9805, DOI: 10.1021/ja502252t.