Carbenes remain an active area of interest for computational chemists, as seen in Chapter 5 of my book. For many carbenes, the triplet is the ground state, and that is true of diphenylcarbene 1. Can solvent play a role in the stability of carbene spin states? Surprisingly, the answer, provided in a recent paper by Sander,1 is yes!
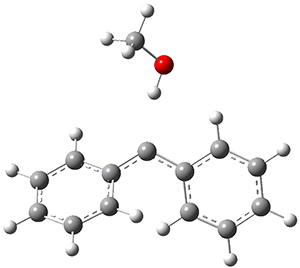
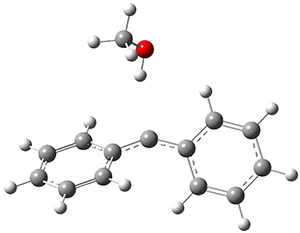
In the gas phase, the singlet-triplet gap of 1 is computed to be 5.62 kcal mol-1 at (U)B3LYP/6-311++G(d,p) (and this reduces to 5.06 at (U)B3LYP+D3/6-311++G(d,p)) with the ground state as a triplet. If a single methanol molecules is allowed to approach 1, then the complex involving the singlet has a short hydrogen bond distance of 1.97 Å but the triplet has a much longer distance of 2.33 Å. These structures are shown in Figure 1. This manifests in a dramatic change in the relative stability, with the singlet complex now 0.26 kcal mol-1 (0.44 with the D3 correction) lower in energy than the triplet.
|
Singlet-1:methanol |
Triplet-1:methanol |
Figure 1. (U)B3LYP/6-311++G(d,p) optimized geometries of the compelxes of methanol with singlet or triplet 1.
IR spectroscopy of 1 in an argon matrix doped with a small amount of methanol confirms the presence of the singlet carbene, and detailed description of the difference in the reactivities of the singlet and triplet are provided.
References
(1) Costa, P.; Sander, W. "Hydrogen Bonding Switches the Spin State of Diphenylcarbene from Triplet to Singlet," Angew. Chem. Int. Ed. 2014, 53, 5122-5125, DOI: 10.1002/anie.201400176.
InChIs
1: InChI=1S/C13H10/c1-3-7-12(8-4-1)11-13-9-5-2-6-10-13/h1-10H
InChIKey=XMGMFRIEKMMMSU-UHFFFAOYSA-N