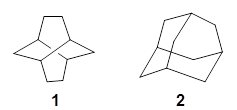
Twistane 1 is a more strained isomer of adamantane 2. The structure of 1 is shown in Figure 1.
|
1 |
Figure 1. B3LYP/6-31G(d) optimized structure of 1.
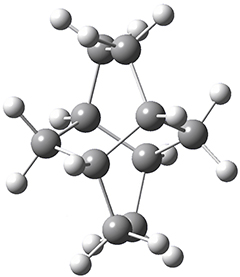
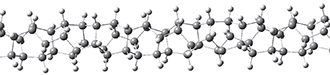
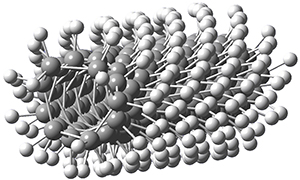
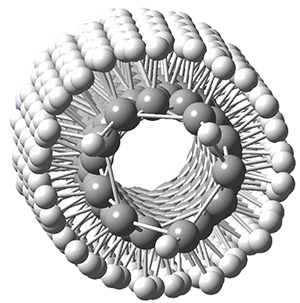
Adamantane is the core structure of diamond, which can be made by appending isobutene groups onto adamantane. In an analogous fashion, twistane can be extended in a linear way by appending ethano groups in a 1,4-bridge. Allen, Schreiner, Trauner and co-workers have examined this “polytwistane” using computational techniques.1 They examined a (CH)236 core fragment of polytwistane, with the dangling valences at the edges filled by appending hydrogens, giving a C236H242 compound. This compound was optimized at B3LYP/6-31G(d) and shown in Figure 2a. (Note that I have zoomed in on the structure, but by activating Jmol – click on the figure – you can view the entire compound.) A fascinating feature of polytwistane is its helical structure, which can be readily seen in Figure 2b. A view down the length of this compound, Figure 2c, displays the opening of this helical cylinder; this is a carbon nanotube with an inner diameter of 2.6 Å.
|
(a) |
|
|
|
|
Figure 2. B3LYP/6-31G(d) structure of the C236H242 twistane. (a) A zoomed in look at the structure. This structure links to the Jmol applet allowing interactive viewing of the molecule – you should try this! (b) a side view clearly showing its helical nature. (c) A view down the twistane showing the nanotube structure.
Though the molecule looks quite symmetric, each carbon is involved in three C-C bonds, and each is of slightly different length. The authors go through considerable detail about addressing the symmetry and proper helical coordinates of polytwistane. They also estimate a strain energy of about 1.6 kcal mol-1 per CH unit. This modest strain, they believe, suggests that polytwistanes might be reasonable synthetic targets.
References
(1) Barua, S. R.; Quanz, H.; Olbrich, M.; Schreiner, P. R.; Trauner, D.; Allen, W. D. "Polytwistane," Chem. Eur. J. 2014, 20, 1638-1645, DOI: 10.1002/chem.201303081.
InChIs
1: InChI=1S/C10H16/c1-2-8-6-9-3-4-10(8)5-7(1)9/h7-10H,1-6H2
InChIKey=AEVSQVUUXPSWPL-UHFFFAOYSA-N
2: InChI=1S/C10H16/c1-7-2-9-4-8(1)5-10(3-7)6-9/h7-10H,1-6H2
InChIKey=ORILYTVJVMAKLC-UHFFFAOYSA-N

Henry Rzepa responded on 01 Jun 2014 at 1:29 am #
Here is an interesting observation. Thanks to Steve making available the 3D coordinates of these structures via Jmol (which oddly works for me on some computers and fails on others; Java continues to be a mystery), I downloaded the polytwistane coordinates and fed them to a visualiser. This happens to have “stereochemistry” turned on, and so I quickly realised that EVERY carbon in this molecule is stereogenic, i.e. assigned either R or S in the CIP convention. Surely there cannot be many hydrocarbons (or indeed non-hydrocarbons) for which this is true?
My main point is that EVERYONE who reports molecular properties should make their coordinates available via the “1-click” route (as opposed to the very much not 1-click procedure of acquiring the supporting information and then grappling with a PDF file to try to extract the coordinates). Such “1-click” procedures make it trivially fast to eg discover what I did above (no more than 1 minute or less).
I know Steve has questioned whether the bother of using Jmol (actually the bother of using Java) makes all this worthwhile. Yes it does! Don’t stop Steve!!
Henry Rzepa responded on 01 Jun 2014 at 1:49 am #
On the theme of chirality, I am interested to find that the ORP (optical rotatory power) of (R,R,R,R)-twistane itself ([α]D) is calculated to be +408° (doi: sz9). The VCD spectrum is equally prominent in the C-H region (doi: sz8).
One might speculate about the behaviour of the chiroptical properties as one extends the polymer length of such molecules.
Steven Bachrach responded on 02 Jun 2014 at 4:31 pm #
I agree with Henry that having a way for authors to deposit “1-click” structures is the way to go! (Copying-and-pasting coordinates from pdf is a pain and often requires considerable massaging.)
This polytwistane molecule is just so visually interesting one really has to play with it using some interactive viewer!