The structure of [2,2]paracyclophane 1 has been somewhat controversial for some time. Early x-ray structures indicated that the molecule was quite symmetric, D2h with the phenyl rings and the ethyl bridges eclipsed. Subsequent low-T experiments suggested a lower symmetry form D2 with a twist that relieves some of the unfavorable eclipsing interactions in the ethano bridges. High-level computations by Grimme1 and then some by myself2 indicated that the D2 structure is the lowest energy conformation, with however a low barrier through the D2h structure.
The suggestion of the D2 minimum was vehemently criticized by Dodziuk, et al. on the basis of NMR analysis.3
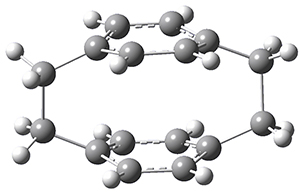
Now, a low temperature x-ray experiment of 1 brings clarity to the situation.4 (The introduction provides a nice summary of the previous 70 year history regarding the structure of 1.) At temperatures below 45 K, 1 is found as a single structure of D2 symmetry (with space group P4n2). The structure is shown in Figure 1. A phase change occurs at about 45 K, and above 60 K the crystal has P42/mnm symmetry. The structure of 1 at the high temperature appears as D2h with somewhat broader thermal motion of the ethano carbons than the phenyl carbons. The low T structure is in excellent accord with the previous theoretical studies, and the phase transition helps bring into accord all of the previous x-ray crystallographic work.
Figure 1. X-ray structure at 15K of 1.
References
(1) Grimme, S. "On the Importance of Electron Correlation Effects for the π-π Interactions in Cyclophanes," Chemistry Eur. J. 2004, 10, 3423-3429, DOI: 10.1002/chem.200400091.
(2) Bachrach, S. M. "DFT Study of [2.2]-, [3.3]-, and [4.4]Paracyclophanes: Strain Energy, Conformations, and Rotational Barriers," J. Phys. Chem. A 2011, 115, 2396-2401, DOI: 10.1021/jp111523u.
(3) Dodziuk, H.; Szymański, S.; Jaźwiński, J.; Ostrowski, M.; Demissie, T. B.; Ruud, K.; Kuś, P.; Hopf, H.; Lin, S.-T. "Structure and NMR Spectra of Some [2.2]Paracyclophanes. The Dilemma of [2.2]Paracyclophane Symmetry," J. Phys. Chem. A 2011, 115, 10638-10649, DOI: 10.1021/jp205693a.
(4) Wolf, H.; Leusser, D.; R. V. Jørgensen, M.; Herbst-Irmer, R.; Chen, Y.-S.; Scheidt, E.-W.; Scherer, W.; Iversen, B. B.; Stalke, D. "Phase Transition of [2,2]-Paracyclophane – An End to an Apparently Endless Story," Chem. Eur. J. 2014, 20, 7048–7053, DOI: 10.1002/chem.201304972.
InChIs
1: InChI=1S/C16H16/c1-2-14-4-3-13(1)9-10-15-5-7-16(8-6-15)12-11-14/h1-8H,9-12H2
InChIKey=OOLUVSIJOMLOCB-UHFFFAOYSA-N

Henry Rzepa responded on 01 Jun 2014 at 1:07 am #
This brings to mind another similar controversy; [18]-annulene. Here too, correlation and dispersion are important. The controversy is between crystallography (which appears to show a D3d-symmetric structure with no bond alternation) and NMR (which strongly suggests a bond-alternating structure of C2 symmetry).
I gather that since there is very little [18]-annulene in existence, no further experiments to resolve this divergence have emerged recently. See doi: c5fdv9.
Henry Rzepa responded on 06 Jun 2014 at 3:18 am #
I have taken a look at another famous, perhaps even at one stage controversial property of benzene rings, the value of the so-called Kekulé vibrational mode. The point of interest was how stacking two benzene rings on top of each other might affect the mode, and whether the bending of these rings and their spin state affects things.
Kekulé’s vibration: A modern example of its use. « Henry Rzepa's blog responded on 08 Jul 2015 at 10:46 am #
[…] to a recent molecule known as [2.2]paracyclophane. The idea was sparked by Steve Bachrach’s latest post, where the “zero-point” structure of the molecule has recently been clarified as having […]