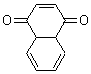
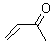
Jorgensen reports an enhanced QM/MM and ab initio study of the rate enhancement of Diels-Alder reactions in various solvents.1 This study extends earlier studies that he and others have done, many of which are discussed in Chapter 6.2 of the book. In this study, he reports QM/MM computations using the PDDG/PM3 method for the QM component, and MP2 computations incorporating CPCM to account for bulk solvent effects.
The major advance in methodology in this paper is performing a two-dimensional potential of mean force analysis where these two dimensions correspond to the forming C-C distances. In addition, computations were done for water, methanol, acetonitrile and hexane as solvents. Highlights of the results are listed in Table 1.
Table 1. Computed bond asynchronicitya and activation energyb (kcal/mol) for the Diels-Alder reaction with cyclopentadiene.
|
|
||||||||
|
|
Gas |
Gas |
water |
methanol |
hexane |
|||
|
dienophile |
Δr |
Δr |
Δr |
ΔG‡ |
Δr |
ΔG‡ |
Δr |
ΔG‡ |
|
|
||||||||
|
|
-0.01 |
0.00 |
0.03 |
26.0 |
0.03 |
29.2 |
-0.03 |
31.1 |
|
|
0.61 |
0.10 |
0.33 |
32.2 |
0.26 |
36.4 |
||
|
|
||||||||
|
aDifference in the lengths of the forming C-C bonds, in Å. bExperimental values in parantheses. |
||||||||
The semi-empirical method underestimates the asynchronicity of these gas-phase Diels-Alder TSs. However, with inclusion of the solvent, the computations do indicate a growing asynchronicity with solvent polarity, This is associated with the ability of the solvent, especially protic solvents, to preferentially hydrogen bond to the carbonyl in the TS.
In terms of energetics, in must first be pointed out that the computations dramatically overestimate the activation barriers. However, the relative trends are reproduced: the barrier increases from water to methanol to acetonitrile to hexane. Jorgensen also computed the activation barriers at MP2/6-311+G(2d,p) with CPCM using the CBS=QB3 gas phase geometries. Some of these results are listed in Table 2. The results for water are in outstanding agreement with experiment. However, the results for the other solvents are poor, underestimating the increase in barrier in moving to the more polar solvent.
Table 2. MP2/6-311+G(2d,p)/CPCM values for ΔG‡ (kcal/mol).
|
|
||||||||
|
dienophile |
water |
methanol |
hexane |
|||||
|
|
16.7 |
17.9 |
18.4 |
|||||
|
|
19.5 |
20.9 |
|
|||||
|
|
||||||||
Bottom line, the conclusions of this study are in agreement with the earlier studies, namely that the hydrophobic effect (better may be the enforced hydrophobic interaction) and greater hydrogen bonding in the TS (both more and stronger hydrogen bonds) account for the rate acceleration of the Diels-Alder reaction in water.
References
(1) Acevedo, O.; Jorgensen, W. L., "Understanding Rate Accelerations for Diels-Alder Reactions in Solution Using Enhanced QM/MM Methodology," J. Chem. Theory Comput. 2007, 3, 1412-1419, DOI: 10.1021/ct700078b.