Jacobsen reports on another application of thiourea-based organocatalysts, this time for the
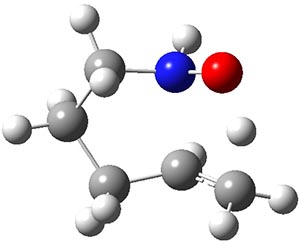
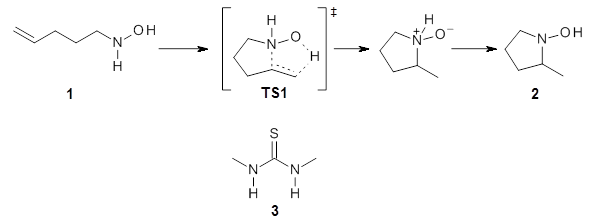
catalysis of hydroamination.1 To support the synthetic effort, he examined the uncatalyzed intramolecular hydroamination that takes 1, through TS1 into product 2. The geometry of TS1 optimized at B3LYP/6-31+G(d,p) is shown in Figure 1. The computed barrier for this reaction is 22.2 kcal mol-1. Using a model thiourea as the catalyst (MeHN)2C=S, 3), Jacobsen locates a
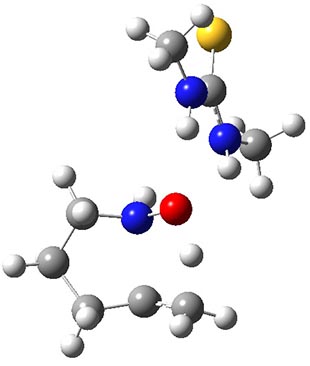
catalyzed transition state TS2 shown in Figure 1. The activation barrier for this catalyzed reaction is 19.1 kcal mol-1, suggesting that a thiourea can afford a real catalytic effect.
|
TS1 |
TS2 |
Figure 1. B3LYP/6-31+G(d,p) optimized geometries of TS1 and TS2(the catalyzed transition state).
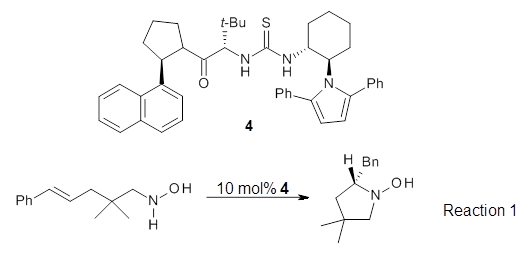
Jacobsen then goes on to show that 4 can act as both an excellent catalyst for the hydroamination reaction along with inducing significant enantioselectivity. An example is Reaction 1, where 10 mol% of catalyst 3 gives an overall yield of 83% and an ee of 91%, while in the absence of catalyst the yield is only 8%.
References
(1) Brown, A. R.; Uyeda, C.; Brotherton, C. A.; Jacobsen, E. N. "Enantioselective Thiourea-Catalyzed Intramolecular Cope-Type Hydroamination," J. Am. Chem. Soc. 2013, 135, 6747-6749, DOI: 10.1021/ja402893z.
InChIs
1: InChI=1S/C5H11NO/c1-2-3-4-5-6-7/h2,6-7H,1,3-5H2
InChIKey=JUMXQRNWLGIKEI-UHFFFAOYSA-N
2: InChI=1S/C5H11NO/c1-5-3-2-4-6(5)7/h5,7H,2-4H2,1H3
InChIKey=YVBPNYXAQNAMLH-UHFFFAOYSA-N
3: InChI=1S/C3H8N2S/c1-4-3(6)5-2/h1-2H3,(H2,4,5,6)
InChIKey=VLCDUOXHFNUCKK-UHFFFAOYSA-N
4: InChI=1S/C44H49N3OS/c1-44(2,3)42(41(48)36-25-15-24-35(36)34-23-14-21-30-16-10-11-22-33(30)34)46-43(49)45-37-26-12-13-27-40(37)47-38(31-17-6-4-7-18-31)28-29-39(47)32-19-8-5-9-20-32/h4-11,14,16-23,28-29,35-37,40,42H,12-13,15,24-27H2,1-3H3,(H2,45,46,49)/t35-,36?,37-,40-,42-/m1/s1
InChIKey=OJMZMPGOFWBKAF-FDGFXIECSA-N