Alonso and coworkers have again (see this post employed laser-ablation molecular-beam Fourier-transform microwave (LA-MB-MW)spectroscopy to discern the gas phase structure of an important biological compound: cytosine.1 They identified five tautomers of cytosine 1-5. Comparison between the experimental and computational (MP2/6-311++G(d,p) microwave rotational constants and nitrogen nuclear quadrupole coupling constants led to the complete assignment of the spectra. The experimental and calculated rotational constants are listed in Table 1.
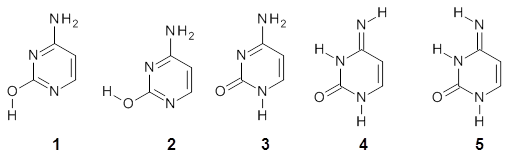
Table 1. Rotational constants (MHz) for 1-5.
|
|
1 |
2 |
3 |
4 |
5 |
|||||
|
|
Expt |
calc |
Expt |
calc |
Expt |
calc |
Expt |
calc |
Expt |
calc |
|
A |
3951.85 |
3934.5 |
3889.46 |
3876.5 |
3871.55 |
3856.0 |
3848.18 |
3820.1 |
3861.30 |
3844.2 |
|
B |
2008.96 |
1999.1 |
2026.32 |
2014.7 |
2024.98 |
2012.3 |
2026.31 |
2019.0 |
2011.41 |
1999.7 |
|
C |
1332.47 |
1326.8 |
1332.87 |
1326.9 |
1330.34 |
1323.3 |
1327.99 |
1324.0 |
1323.20 |
1318.4 |
The experimental and computed relative free energies are listed in Table 2. There is both not a complete match of the relative energetic ordering of the tautomers, nor is there good agreement in their magnitude. Previous computations2 at CCSD(T)/cc-pVQZ//CCSD//cc-pVTZ are in somewhat better agreement with the gas-phase experiments.
Table 2. Relative free energies (kcal mol-1) of 1-5.
|
|
expt |
MP2/ |
CCSD(T)/cc-pVQZ// |
|
1 |
0.0 |
0.0 |
0.0 |
|
2 |
0.47 |
0.70 |
0.7 |
|
3 |
0.11 |
1.19 |
0.2 |
|
4 |
0.83 |
3.61 |
0.7 |
|
5 |
|
5.22 |
|
References
(1) Alonso, J. L.; Vaquero, V.; Peña, I.; López, J. C.; Mata, S.; Caminati, W. "All Five Forms of Cytosine Revealed in the Gas Phase," Angew. Chem. Int. Ed. 2013, 52, 2331-2334, DOI: 10.1002/anie.201207744.
(2) Bazso, G.; Tarczay, G.; Fogarasi, G.; Szalay, P. G. "Tautomers of cytosine and their excited electronic states: a matrix isolation spectroscopic and quantum chemical study," Phys. Chem. Chem. Phys., 2011, 13, 6799-6807, DOI:10.1039/C0CP02354J.
InChIs
cytosine: InChI=1S/C4H5N3O/c5-3-1-2-6-4(8)7-3/h1-2H,(H3,5,6,7,8)
InChIKey=OPTASPLRGRRNAP-UHFFFAOYSA-N

Igor Reva responded on 23 Apr 2013 at 12:20 pm #
For those who are interested in cytosine conformers, I call attention to some more publications:
Lapinski, L., Nowak, M.J., Reva, I., Rostkowska, H. and Fausto, R. NIR-laser-induced selective rotamerization of hydroxy conformers of cytosine. Physical Chemistry Chemical Physics, 2010, v. 12, No. 33, pp. 9615-9618. DOI: 10.1039/c0cp00177e.
Lapinski, L., Reva, I., Nowak, M.J. and Fausto, R. Five isomers of monomeric cytosine and their interconversions induced by tunable UV laser light. Physical Chemistry Chemical Physics, 2011, v. 13, No. 20, pp. 9676-9684. DOI: 10.1039/c0cp02812f.
Reva, I., Nowak, M.J., Lapinski, L. and Fausto, R. Spontaneous tunneling and near-infrared-induced interconversion between the amino-hydroxy conformers of cytosine. Journal of Chemical Physics, 2012, v. 136, No. 6, Article Number 064511. DOI: 10.1063/1.3683217.
The title of the second work is self-explanatory. 😉
IR