Here’s one more nice application of computationally-derived NMR chemical shifts towards solving a structure. Fattarusso and co-workers1 identified a component of wormwood called artarborol. COSY and ROESY experiments allowed for deducing four possible diasereomeric structures of artarborol, 1-4.

They then took two computational approaches towards resolving the structure. First, they performed an MM search for low energy conformers of 1-4. These conformers were then screened for those having a dihedral angle of around 90° for the C-8 and C-9 protons, due to a low couple constant for between these protons. Only conformers of 1 and 3 satisfied this criterion. An intense couple of the H-1 and H-5 protons indicated a transannular arrangement, and only conformers of 1 satisfy this criterion.
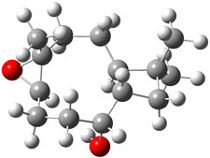
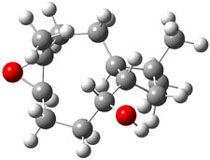
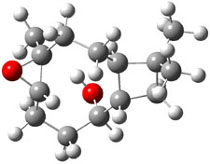
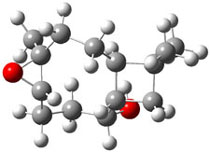
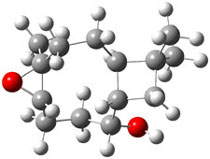
The second computational approach was to optimize some of the low energy conformers of 1 and 3 at mPW1PW91/6-31G(d,p) and compute their 13C chemical shifts. The five low energy conformers, two of 1 and three of 3, are shown in Figure 1. The resulting chemical shifts were averaged according to a Boltzmann distribution. These computed chemical shifts were then fit against the experimental values. The correlation factor for the computed shifts for 1 (r2=0.9997) was much better than that of 3 (r2=0.9713). The average deviation of the chemical shifts (after being corrected using the fitting procedure from the above correlation) was only 0.8ppm for 1 but 2ppm for 3. They therefore conclude that the structure of artarborol is 1.
|
1a |
1b |
|
|
3a |
3b |
3c |
Figure 1. mPW1PW91 optimized conformations of possible artarborol diasteromers.1
References
(1) Fattorusso, C.; Stendardo, E.; Appendino, G.; Fattorusso, E.; Luciano, P.; Romano, A.; Taglialatela-Scafati, O., "Artarborol, a nor-Caryophyllane Sesquiterpene Alcohol from Artemisia arborescens. Stereostructure Assignment through Concurrence of NMR Data and Computational Analysis," Org. Lett., 2007, 9, 2377-2380, DOI: 10.1021/ol070803s.
(2) I thank Professor Ernesto Fattorusso for supplying me with the optimized coordinates of these compounds.
InChI
1: InChI=1/C14H24O2/c1-13(2)8-9-10(13)6-7-14(3)12(16-14)5-4-11(9)15/h9-12,15H,4-8H2,1-3H3/t9-,10-,11-,12-,14+/m0/s1