Just a short update here. In Chapter 5.1.2 we discuss nucleophilic substitution at heteroatoms. Unlike the paradigmatic case for substitution at carbon, which proceeds via the SN2 mechanism. Nucleophilic substitution at second-row atoms (S, Si, P) appears to follow an addition-elimination pathway. Bickelhaupt1 now adds a more thorough computational examination of nucleophilic substitution at phosphorus. He looked at a few identity reactions involving tricoordinate P, namely
X– + PH2X → PH2X + X–
X– + PF2X → PF2X + X–
X– + PCl2X → PCl2X + X–
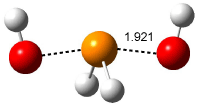
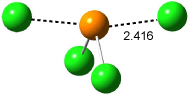
where X is chloride or hydroxide. In all cases the only critical point located on the potential energy surface is for a tetracoordinate intermediate. Shown in Figure 1 are the intermediates for the reaction OH– + PH2OH and Cl– + PCl3. This result is consistent with the studies of nucleophilic substitution at sulfur and silicon.
|
(a) |
(b) |
Figure 1. OLYP/TZ2P optimized intermediate for the reaction (a) OH– + PH2OH
and (b) Cl– + PCl3.
References:
(1) vanBochove, M. A.; Swart, M.; Bickelhaupt, F. M., "Nucleophilic Substitution at Phosphorus (SN2@P): Disappearance and Reappearance of Reaction Barriers," J. Am. Chem. Soc. 2006, 128, 10738-10744, DOI: 10.1021/ja0606529