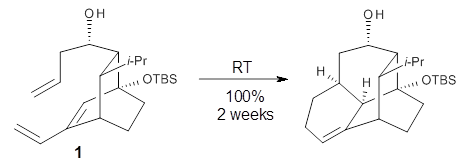
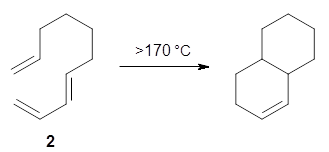
The intramolecular Diels-Alder reaction of 1 occurs slowly, but quantitatively, at room temperature.1 This is unusual as most Diels-Alder cyclizations require heating to typically 200 °C. For example, the related cyclization of 2 requires heating to 170 °C.2 What is the cause for this proximity-induced reaction?
|
|
Reaction 1 |
|
|
Reaction 2 |
|
|
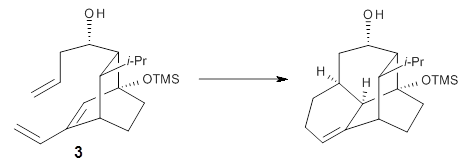
Reaction 3 |
Houk and Baran address this question using a computational approach.3 The Diels-Alder reaction of 2 and a simplified analogue of 1, namely 3, were computed at CPCM/M06-2x/6-311+G(d,p)//B3LYP/6-31G(d). The optimized transition states for the reaction of 2 and 3 are shown in Figure 1. The free energy of activation of 3 is 5.4 kcal mol-1 lower in energy than the free energy of activation of 2. This is consistent with the much faster reaction of 1 than 2 observed in the experiment.
|
TS2 |
TS3 |
Figure 1. B3LYP/6-31G(d) for the transition states of Reactions 2 and 3.
Partitioning 3 into fragments allows Houk and Baran to apply the distortion model. They find that the rigid diene in 3 (and thereby 1) accelerates the reaction relative to the more flexible diene of 2. Further, strain relief in going from 3 (and thereby 1) to TS3 (and thereby to TS of reaction 1) and the formation of an intramolecular hydrogen bond leads to the lower activation energy of 3, and therefore of 1.
References
(1) Maimone, T. J.; Voica, A.-F.; Baran, P. S. "A Concise Approach to Vinigrol," Angew. Chem. Int. Ed. 2008, 47, 3054-3056, DOI: 10.1002/anie.200800167.
(2) Diedrich, M. K.; Klärner, F.-G.; Beno, B. R.; Houk, K. N.; Senderowitz, H.; Still, W. C. "Experimental Determination of the Activation Parameters and Stereoselectivities of the Intramolecular Diels−Alder Reactions of 1,3,8-Nonatriene, 1,3,9-Decatriene, and 1,3,10-Undecatriene and Transition State Modeling with the Monte Carlo-Jumping Between Wells/Molecular Dynamics Method," J. Am. Chem. Soc. 1997, 119, 10255-10259, DOI: 10.1021/ja9643331.
(3) Krenske, E. H.; Perry, E. W.; Jerome, S. V.; Maimone, T. J.; Baran, P. S.; Houk, K. N. "Why a Proximity-Induced Diels–Alder Reaction Is So Fast," Org. Lett. 2012, 14, 3016-3019, DOI: 10.1021/ol301083q.
InChIs
1: InChI=1S/C23H40O2Si/c1-10-12-19(24)21-20(16(3)4)18-13-14-23(21,15-17(18)11-2)25-26(8,9)22(5,6)7/h10-11,15-16,18-21,24H,1-2,12-14H2,3-9H3/t18?,19-,20?,21?,23+/m0/s1
InChIKey=NGVNTJGCNDZDEY-RHDCMTSYSA-N
2: InChI=1S/C10H16/c1-3-5-7-9-10-8-6-4-2/h3-5,7H,1-2,6,8-10H2/b7-5+
InChIKey=HXZJJSYHNPCGKW-FNORWQNLSA-N
3: InChI=1S/C20H34O2Si/c1-8-10-18(21)20(15(3)4)14-17-11-12-19(20,13-16(17)9-2)22-23(5,6)7/h8-9,13,15,17-18,21H,1-2,10-12,14H2,3-7H3/t17?,18-,19+,20?/m0/s1
InChIKey=GDQHAOHEZAJKPI-FUFFSDJGSA-N

Henry Rzepa responded on 09 Oct 2012 at 10:18 am #
There is something else odd about norbornene itself (and possibly hence of its reactions). Clemence Corminboeuf (doi: 10.1039/c0cc00601g) has recently reported to have solved the mystery. Apparently, norbornene manifests a significant density redistribution towards an incipient retro-Diels-Alder reaction.
Perhaps the two observations are related?
Ken Houk responded on 22 Oct 2012 at 5:49 pm #
Even more relevant is our study of “factor X” – Huisgen’s name for the unusually high reactivity of norbornene (incidentally there is no norbornene in Baran’s molecule. See our relatively new work on this, done by Steven Lopez in my group: doi: 10.1021/jo301267b).
Also, it is Elizabeth Krenske, a research fellow at the University of Melbourne, moving now to Queensland, who actually did the calculations and analysis so nicely covered here by Bachrach!