Cyclobutadiene is the prototypical antiaromatic compound. McMahon has examined the
effect of ethynyl substitution on this ring, with a long term eye towards the possibility of these types of species being involved in the synthesis of fullerenes.1
All of the possible ethynyl-substituted cyclobutadiene species (1-7) were optimized at B3LYP/6-31G(d) and CCSD/cc-pVDZ in their singlet and triplet states.

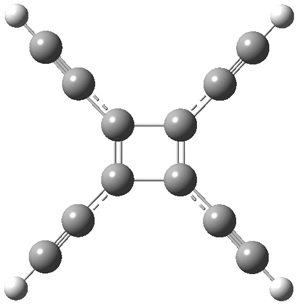
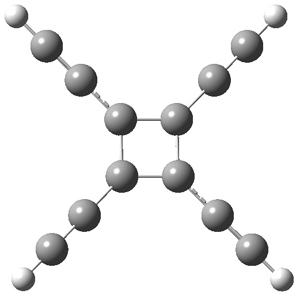
The structures of singlet and triplet 7 are shown in Figure 1. The geometries provided by the two different methods are quite similar. They show a rectangular form for the singlets and a delocalized, nearly square ring for the triplets.
|
7singlet |
7triplet |
Figure 1. CCSD/cc-pVDZ optimized structures of singlet and triplet 7.
The computed singlet-triplet gap decreases with each ethynyl substituent. B3LYP, which overestimates the stability of triplets, predicts that 6 and 7 will be ground state triplets, while CCSD predicts a singlet ground state for all 7 species, with the gap decreasing steadily from 11.5 to 8.2 kcal mol-1, a value that is also probably underestimated.
This change in the singlet-triplet gap reflects a stronger stabilizing effect of each ethynyl group to the cycnobutadiene ring for the triplet than for the singlet state. This is seen in the homodesmotic stabilization energies.
Lastly, NICS(1)zz values are positive for all of the singlets and negative for the triplets. The positive values for the singlets reflect their antiaromatic character, also seen in the alternant bond distances around the ring. The NICS values of the singlets decrease with increasing substitution. The negative NICS values of the triplets reflects aromatic character, as seen in the non-alternant distances around the ring. Interestingly, the triplet NICS values decrease with increasing ethynyl substitution, suggesting decreased aromaticity, even though the homodesmotic reactions suggest increasing stabilization with substitution.
References
(1) Esselman, B. J.; McMahon, R. J., "Effects of Ethynyl Substitution on Cyclobutadiene," J. Phys. Chem. A 2012, 116, 483-490, DOI: 10.1021/jp206478q
InChIs
1: InChI=1/C4H4/c1-2-4-3-1/h1-4H
InChIKey=HWEQKSVYKBUIIK-UHFFFAOYAI
2: InChI=1/C6H4/c1-2-6-4-3-5-6/h1,3-5H
InChIKey=XFHXCHFBSJDBGT-UHFFFAOYAG
3: InChI=1/C8H4/c1-3-7-5-6-8(7)4-2/h1-2,5-6H
InChIKey=YSSBCMLQKUIAEP-UHFFFAOYAI
4: InChI=1/C8H4/c1-3-7-5-8(4-2)6-7/h1-2,5-6H
InChIKey=IRAQOGPKMAXTQY-UHFFFAOYAN
5: InChI=1/C8H4/c1-3-7-5-6-8(7)4-2/h1-2,5-6H
InChIKey=YSSBCMLQKUIAEP-UHFFFAOYAI
6: InChI=1/C10H4/c1-4-8-7-9(5-2)10(8)6-3/h1-3,7H
InChIKey=BVCIPPXDFXWTJR-UHFFFAOYAQ
7: InChI=1/C12H4/c1-5-9-10(6-2)12(8-4)11(9)7-3/h1-4H
InChIKey=HEUYILYXFVQGOW-UHFFFAOYAO