Once again the Singleton group reports experiments and computations that require serious reconsideration of our notions of reaction mechanisms.1 In this paper they examine the reaction of dichloroketene with labeled cis-2-butene. With 13C at the 2 position of 2-butene, two products are observed, 1 and 1’, in a ratio of 1’:1 = 0.993 ± 0.001. This is the opposite what one might have imagined based on the carbonyl carbon acting as an electrophile.
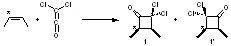
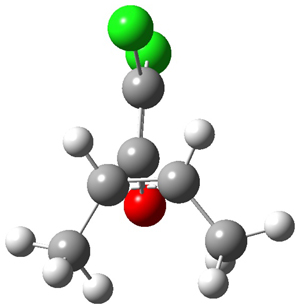
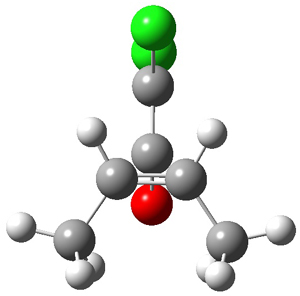
The first interesting item is that B3LYP/6-31+G** fails to predict the proper structure of the transition state. It predicts an asymmetric structure 2, shown in Figure 1, while MPW1k/6-31+G**, M06, and MP2 predict a Cs transition structure 3. The Cs TS is confirmed by a grid search of M06-2x geometries with CCSD(T)/6-311++G88/PCM(CH2Cl2) energies.
|
2 |
3 |
Figure 1. Optimized TSs 2 (B3LYP/6-31+G**) and 3 (MPW1K/6-31+G**).
The PES using proper computational methods is bifurcating past TS 3, falling downhill to product 1 or 1’. Lying on the Cs plane is a second transition state that interconverts 1 and 1’. On such a surface, conventional transition state theory would predict equal amounts of 1 and 1’, i.e. no isotope effect! So they must resort to a trajectory study – which would be impossibly long if not for the trick of making the labeled carbon super-heavy – like 28C,44C, 76C and 140C and then extrapolating back to just ordinary 13C. These trajectories indicate a ratio of 1’:1 of 0.990 in excellent agreement with the experimental value of 0.993.
Interestingly, most trajectories recross the TS, usually by reaching into the region near the second TS. However, the recrossing decreases with increasing isotopic mass, and this leads to the isotope effect. It turns out the vibrational mode 3 breaks the Cs symmetry; movement in one direction along mode 3 has no mass dependence but in the opposite direction, increased mass leads to decreased recrossing – or put in another way, in this direction, increased mass leads more often to product.
But one can understand this reaction from a statistical point of view as well. If one looks at the free energy surface, there is a variational TS near 3, but then there is a second set of variational transition states (one leading to 1 and one to 1’) which are associated with the formation of the second C-C bond. In a sense there is an intermediate past 3 that leads to two entropic barriers, one on a path to 1 and one on the path to 1’. RRKM using this model gives a ratio of 0.992 – again in agreement with experiment! It is as Singleton notes “perplexing”; how do you reconcile the statistical view with the dynamical (trajectory) view? Singleton has no full explanation.
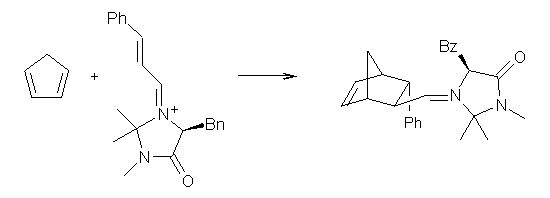
Lastly, they point out that a similar situation occurs in the organocatalyzed Diels-Alder reaction of MacMillan shown below.2 (This reaction is also discussed in a previous post.) Now Singleton finds that the “substituent effects, selectivity, solvent effects, isotope effects and activation parameters” are all dictated by a second variational TS far removed from the conventional electronic TS.
References
(1) Gonzalez-James, O. M.; Kwan, E. E.; Singleton, D. A., "Entropic Intermediates and Hidden Rate-Limiting Steps in Seemingly Concerted Cycloadditions. Observation, Prediction, and Origin of an Isotope Effect on Recrossing," J. Am. Chem. Soc. 2012, 134, 1914-1917, DOI: 10.1021/ja208779k
(2) Ahrendt, K. A.; Borths, C. J.; MacMillan, D. W. C., "New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels-Alder Reaction," J. Am. Chem. Soc. 2000, 122, 4243-4244, DOI: 10.1021/ja000092s.
InChIs
2-butene: InChI=1/C4H8/c1-3-4-2/h3-4H,1-2H3/b4-3-
InChIKey=IAQRGUVFOMOMEM-ARJAWSKDBO
Dichloroketene: InChI=1/C2Cl2O/c3-2(4)1-5
InChIKey=TVWWMKZMZALOFP-UHFFFAOYAY
1 (no isotope): InChI=1/C6H8Cl2O/c1-3-4(2)6(7,8)5(3)9/h3-4H,1-2H3/t3-,4+/m0/s1
InChIKey=BAEYWHUXGUIZSP-IUYQGCFVBH

Rafael R. Pappalardo responded on 10 Mar 2012 at 4:09 am #
Just a quick note: the year should be 2012 in the reference to Singleton paper.
Steven Bachrach responded on 13 Mar 2012 at 7:56 am #
Rafael- Thanks for the comment – I have updated the post to now include the correct year!