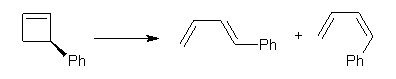
Torquoselectivity rules (discussed in Chapter 3.5 of my book) indicate that 3-phenylcyclobutene will ring-open to give the outward rotated product (Reaction 1). Houk and Tang report a seeming contradiction, namely the ring opening of 1 gives only the inward product 3 (Reaction 2).1
|
|
Reaction 1 |
|
|
Reaction 2 |
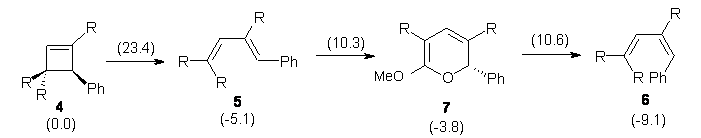
B3LYP/6-31G* computations on the ring-opening of 4 indicate that the activation barrier for the outward path (leading to 5) is nearly 8 kcal mol-1 lower than the barrier for the inward path (leading to 6, see Reaction 3). This is consistent with torquoselectivity rules, but what is going on in the experiment?
|
|
Reaction 3 |
In the investigation of the isomerization of the outward to inward pathway, they discovered a low-energy pyran intermediate 7. This led to the proposal of the mechanism shown in Reaction 3. The highest barrier is for the electrocyclization that leads to the outward product 5. The subsequent barriers – the closing to the pyran 7 and then the torquoselective ring opening to 6 – are about than 13 kcal mol-1 lower in energy than for the first step. The observed product is the thermodynamic sink. And the nice thing about this mechanism is that torquoselection is preserved.
|
|
References
(1) Um, J. M.; Xu, H.; Houk, K. N.; Tang, W., "Thermodynamic Control of the Electrocyclic
Ring Opening of Cyclobutenes: C=X Substituents at C-3 Mask the Kinetic Torquoselectivity," J. Am. Chem. Soc. 2009, 131, 6664-6665, DOI: 10.1021/ja9016446.
InChIs
4: InChI=1/C16H16O6/c1-20-13(17)11-9-16(14(18)21-2,15(19)22-3)12(11)10-7-5-4-6-8-10/h4-9,12H,1-3H3
InChIKey=VBOGEHVOAGDMNG-UHFFFAOYAR
5: InChI=1/C16H16O6/c1-20-14(17)12(9-11-7-5-4-6-8-11)10-13(15(18)21-2)16(19)22-3/h4-10H,1-3H3/b12-9-
InChIKey=PZRWKBUUAFMPBC-XFXZXTDPBF
6: InChI=1/C16H16O6/c1-20-14(17)12(9-11-7-5-4-6-8-11)10-13(15(18)21-2)16(19)22-3/h4-10H,1-3H3/b12-9+
InChIKey=PZRWKBUUAFMPBC-FMIVXFBMBS
7: InChI=1/C16H16O6/c1-19-14(17)11-9-12(15(18)20-2)16(21-3)22-13(11)10-7-5-4-6-8-10/h4-9,13H,1-3H3/t13-/m0/s1
InChIKey=QSJZITDSTPMCEM-ZDUSSCGKBG