Matt Seibert pointed out to me a paper of his related to a previous blog post that also deals with the allene-yne thermal [2+2] cyclization. (My apologies to Matt and Dean for overlooking this paper!) Tantillo and Brummond looked at the system with various saturated tethers between these functional groups.1 For example, UB3LYP/6-31+G(d,p) study of the cyclization of 1 indicates two possible paths, where the 5-member ring is formed first, or where the 7 member ring is formed first. The relative energies of the TSs and intermediates are shown in Figure 1. (Note that there are actually two intermediates on the first pathway, differing in the orientation terminal methyne hydrogen.) The closure to the smaller ring first is favored due to the allylic stabilization of the radical intermediate on this pathway.
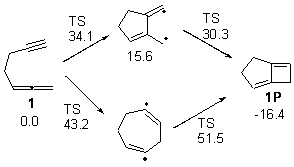
Figure 1. Relative energies of TSs and critical point in the cyclization of 1.
Next, they examined the regioselectivity for the inner or outer double bond of the allene in 2. For the reaction with the outer double bond, the 6 member ring is formed first. For the reaction with the inner double bond, the 7-member ring is formed first, and this pathway has a higher barrier than the other. The preference for the reaction with the terminal double bond is consistent with experiments.
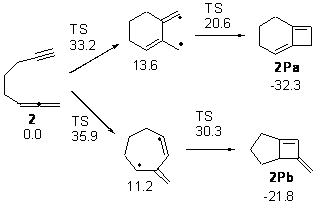
Figure 2. Relative energies of TSs and critical point in the cyclization of 2.
With potential diradical intermediates, they decided to append a cyclopropyl ring to as a trap. So, for example, the reaction of 3 can lead to the [2+2] product or to a diradical that might be trapped and identified. The computed energies along these two paths are shown in Figure 3. The activation barrier for the closure to the 2+2 product and for ring opening of the cyclopropyl group are nearly identical, so one might expect to observe both processes. Analogues of 3 were prepared and heated; some evidence of the ring opening of the cyclopropyl group was observed.
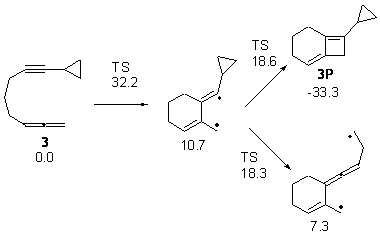
Figure 3. Relative energies of TSs and critical point in the cyclization of 3.
References
(1) Siebert, M. R.; Osbourn, J. M.; Brummond, K. M.; Tantillo, D. J., "Differentiating Mechanistic Possibilities for the Thermal, Intramolecular [2 + 2] Cycloaddition of Allene-Ynes," J. Am. Chem. Soc., 2010, 132, 11952-11966, DOI: 10.1021/ja102848z
InChIs
1: InChI=1/C7H8/c1-3-5-7-6-4-2/h1,6H,2,5,7H2
InChIKey=IFOUEVYNVOTODB-UHFFFAOYAI
1P: InChI=1/C7H8/c1-2-6-4-5-7(6)3-1/h2,5H,1,3-4H2
InChIKey=UCCCTNVCSUBFQC-UHFFFAOYAW
2: InChI=1/C8H10/c1-3-5-7-8-6-4-2/h1,6H,2,5,7-8H2
InChIKey=SXDPESVCUVUSMY-UHFFFAOYAU
2Pa: InChI=1/C8H10/c1-2-4-8-6-5-7(8)3-1/h3,6H,1-2,4-5H2
InChIKey=TYATUGPSJUHEJF-UHFFFAOYAU
2Pb: InChI=1/C8H10/c1-6-5-7-3-2-4-8(6)7/h5,8H,1-4H2
InChIKey=MKCUOZNLKWXQFA-UHFFFAOYAC
3: InChI=1/C11H14/c1-2-3-4-5-6-7-8-11-9-10-11/h3,11H,1,4-6,9-10H2
InChIKey=XLOBKJCWEUMVHP-UHFFFAOYAK
3P: InChI=1/C11H14/c1-2-4-10-9(3-1)7-11(10)8-5-6-8/h3,8H,1-2,4-7H2
InChIKey=NLALHZZTFZLGMN-UHFFFAOYAM

Alex Thom responded on 29 Aug 2011 at 2:36 pm #
I’m rather skeptical of these sorts of calculations, particularly in terms of the errors implicit in the computational methods – which are not spelled out – and quite what conclusions one can sensibly draw in light of those unknown errors.
I would expect significant errors in UB3LYP energetics for multiple bond breaking/forming transition states. The paper talks of testing the results against UMP2 and UCCSD(T) and then discards these because of spin contamination, opting to believe the UB3LYP on the basis of lesser spin contamination. In any case, the wavefunction-based correlated calcalations are performed in such a small basis set as to have little predictive value. What sort of systematic errors would you expect to see in these calculations, and are they usually similar enough between different reaction paths to cancel out enough to justify the conclusions?
I’d also expect (purely from chemical intuition) solvent effects to be incredibly important (especially to the free energy), but these are brushed away in a few of continuum solvent calculations – what sort of errors should we expect in these?
The conclusions of the paper are that the calculations “provide evidence” for one pathway over another, but I’d be interested to know whether putting all those errors estimates together still allows one to draw the same conclusions.