The search for a stable molecule containing a hypercoordinated carbon atom may finally be over. Abboud and Yáñez1 report mass spectra and computations on the unusual cation Si(CH3)3CH3Si(CH3)3+ (1). Using low pressure FT-ICR, upon ionization of Si(CH3)4 (TMS) they observe a small signal with m/z 161.12, which corresponds to the mass of 1+. When Si(CH3)4 and Si(CD3)4 are mixed, introduced into the spectrometer and ionized, the mixed isotopomer of 1+ is observed, and scrambling to the CH3 and CD3 groups occurs.
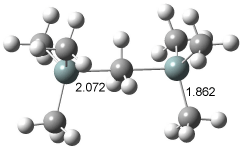
The geometry of 1+ was optimized at B3LYP/6-311+G(3df,2pd) and QCISD/6-311+G(d,p). The latter structure is shown in Figure 1, though the geometry differs little between the two computations. The enthalpy for the dissociation of 1+ into TMS and Si(CH3)3+ is 23.2 kcal mol-1 at QCISD. This compares very well with the experimental2 value of 22.3 kcal mol-1.
Figure 1. QCISD/6-311+G(d,p) optimized structure of 1+.1
The structure has C3h symmetry with the central carbon to silicon distance of 2.071 Å, a bit more than a 10% increase over the length of a typical C-Si bond. Topological electron density analysis indicates a bond critical point does connect the central carbon atom with each silicon atom, and the value of the Laplacian of the electron density at this critical point is negative. This analysis strongly suggests that the central carbon atom is pentacoordinate!
Abboud and Yáñez argue that 1+ can be considered as a complex of methyl cation and two Si(CH3)3 radicals. The empty p orbital of the central methyl carbon can then interact with the radical-bearing orbital on each silicon, forming a three-center two-electron bonding molecular orbital (Scheme 1). The positive charge is delocalized, with the central methyl group having a charge of +0.26 while the charge on each Si(CH3)3 groups is +0.37.
Scheme 1.

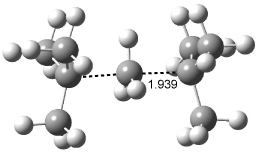
Though not discussed in this paper, the all carbon analogue, namely C(CH3)3CH3C(CH3)3+
is not a stable structure when restricted to have C3h symmetry (see Figure 2). Rather, this geometry corresponds to the transition state for the transfer of a methyl group from one C(CH3)3 group to the other.
Figure 1. B3LYP/6-311+G(d,p) optimized geometry of C(CH3)3CH3C(CH3)3+.
References
(1) Daacute;valos, J. Z.; Herrero, R.; Abboud, J.-L. M.; Mó, O.; Yáñez, M., "How Can a Carbon Atom Be Covalently Bound to Five Ligands? The Case of Si2(CH3)7+," Angew. Chem. Int. Ed. 2007, 46, 381-385, DOI: 10.1002/anie.200601164
(2) Wojtyniak, A. C. M.; Li, X.; Stone, J. A., "The Formation of CH3)7Si2+ in (CH3)4Si/CH4 Mixtures and CH3−Exchange Reactions between (CH3)4Si, (CH3)4Ge, and (CH3)4Sn Studied by High Pressure Mass Spectrometry," Can. J. Chem. 1987, 65, 2849-2854,