We have all learned about aromatic substitution as proceeding via the following mechanism
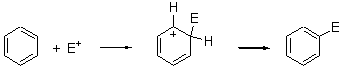
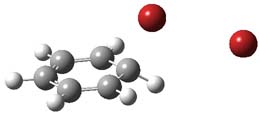
(Worse yet – many of us have taught this for years!) Well, Galabov, Zou, Schaefer and Schleyer pour a whole lot of cold water on this notion in their recent Angewandte article.1 Modeling the reaction of benzene with Br2 and using B3LYP/6-311+G(2d,2p) for both the gas phase and PCM simulating a CCl4 solvent, attempts to locate this standard intermediate led instead to a concerted substitution transition state TS1 (see Figure 1).
|
TS1 |
Figure 1. PCM/B3LYP/6-311+G(2d,2p) optimized transitin state along the concerted pathway
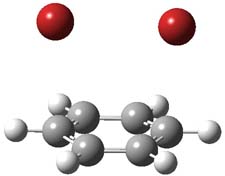
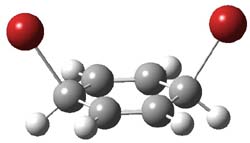
However, this is not the lowest energy pathway for substitution. Rather and addition-elimination pathway is kinetically preferred. In the first step Br2 adds in either a 1,2 or 1,4 fashion to form an intermediate. The lower energy path is the 1,4 addition, leading to P3. This intermediate then undergoes a syn,anti-isomerization to give P5. The last step is the elimination of HBr from P5 to give the product, bromobenzene. This mechanism is shown in Scheme 2 and the critical points are shown in Figure 3.
Scheme 1

|
TS3 |
P3 |
|
TS6 |
P5 |
|
TS9 |
|
Figure 2. PCM/B3LYP/6-311+G(2d,2p) optimized critical points along the addition-elimination pathway
The barrier for the concerted substitution process through TS1 is 41.8 kcal mol-1 (in CCl4) while the highest barrier for the addition-elimination process is through TS3 of 39.4 kcal mol-1.
Now a bit of saving grace is that in polar solvents, acidic solvents and/or with Lewis acid catalysts, the intermediate of the standard textbook mechanism may be competitive.
Textbook authors – please be aware!
References
(1) Kong, J.; Galabov, B.; Koleva, G.; Zou, J.-J.; Schaefer, H. F.; Schleyer, P. v. R., "The Inherent Competition between Addition and Substitution Reactions of Br2 with Benzene and Arenes," Angew. Chem. Int. Ed. 2011, 50, 6809-6813, DOI: 10.1002/anie.201101852
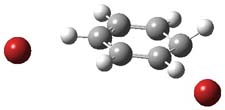
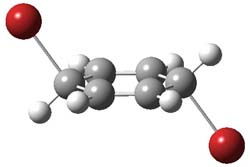
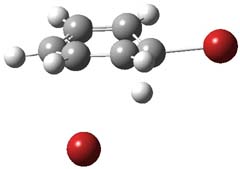

Henry Rzepa responded on 29 Sep 2011 at 8:31 am #
“the highest barrier for the addition-elimination process is through TS3 of 39.4 kcal mol-1.”? That is a hell of a barrier, well beyond normal thermal accessibility. Oh, is that a free energy barrier or an enthalpy barrier? We need to know (it is after all a bimolecular reaction)!
I agree that aromatic electrophilic substitution is not always what it seems. Some time ago, I noted that the “Wheland intermediate” could turn into a Wheland transition state. And sometimes the “intermediate” is actually the stable species!.
Alan Shusterman responded on 04 Oct 2011 at 11:39 pm #
I haven’t read their paper, but thanks for the lucid summary.
Do you know why they studied the uncatalyzed reaction? Textbooks typically tell students that benzene is unreactive towards bromine and a Lewis acid catalyst is needed. This is consistent, at least, with the high calc’d barrier found by these authors; perhaps the textbooks are on safe ground after all?
Steven Bachrach responded on 05 Oct 2011 at 7:14 am #
It is an interesting point about the catalyst. My guess is that the authors omitted based on the size of the problem – but this is a rather weak excuse. Including the catalyst is worth investigation – and goes along Henry Rzepa’s concern to be complete (see his post).
Yong Boon responded on 12 Jun 2018 at 10:10 pm #
But how does the elimination even take place in the last step in the proposed alternative mechanism? It seems to me that there are no available alpha hydrogens anti to the C-Br bond. I would think that the adjacent H-C bonds would also be very hard to break considering that those are sp2 carbons.