The combined supersonic jet expansion and Fourier transform microwave spectroscopy provides an excellent opportunity for the synergistic workings of experiments and computations. This is nicely demonstrated in the study of 1-methyl-4-piperidone.1
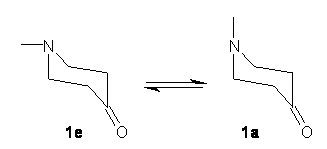
The careful microwave study allows for the full structural characterization of the equatorial form 1e along with obtaining a good deal of information concerning the axial form 1a. To help evaluate the experimental data, the authors have optimized the structure of the two isomers at MP2, B3LYP and M06-2x using the 6-311++G(d,p) basis set.
The rotational parameters computed with the three methods are in very fine agreement with the experimental values. Of particular note is that the three computations predict a different sign for the nuclear quadrupole coupling tensor elements χaa and χbb, and this is observed in the experiment as well. It is perhaps the critical identifier of the axial isomer. The computed and experimental geometries of 1e are in fine agreement, with the largest deviation of a few degrees in the dihedral angle of the carbonyl to the ring. The experiment suggests an energy difference of 11.9 kJ mol-1, which is corroborated by MP2, B3LYP and M06-2x computations. In fact, these first two methods predict an enthalpy difference within a kJ of the experimental value.
References
(1) Evangelisti, L.; Lesarri, A.; Jahn, M. K.; Cocinero, E. J.; Caminati, W.; Grabow, J.-U., "N-Methyl Inversion and Structure of Six-Membered Heterocyclic Rings: Rotational Spectrum of 1-Methyl-4-piperidone," J. Phys. Chem. A, 2011,
115, 9545–9551, DOI: 10.1021/jp112425w
InChIs
1: InChI=1/C6H11NO/c1-7-4-2-6(8)3-5-7/h2-5H2,1H3
InChIKey=HUUPVABNAQUEJW-UHFFFAOYAT
