While many pericyclic reactions proceed in a concerted fashion, the stepwise pathway is a distinct possibility. Fernandez, Sierra and Torres report on an interesting [8+2] cycloaddition that
is decidedly stepwise, confirmed through trapping of the intermediate zwitterion.1
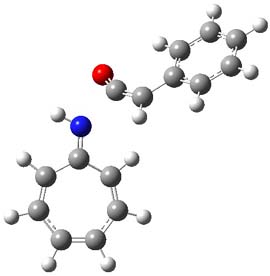
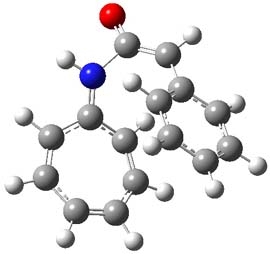
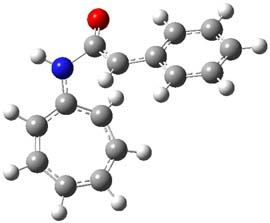
The reaction of 1 with 2 was examined at M06-2x/6-311+G(d) (optimized geometries of the critical points are shown in Figure 1). The first transition state (TS1) has nitrogen acting as a nucleophile, attacking the carbonyl carbon of ketene to give 3. The barrier is 11.6 kcal mol-1, and 3 lies 0.7 kcal mol-1 above reactants. While 3 might be described with a tropyllium cation resonance structure, the ring is in fact non-planar and both the NICS(0) and NICS(1)zz values are positive. The ring is therefore antiaromatic, consistent with the endoergonicity of this step. Closure of the zwitterion through TS2 leads to the formal [8+2] product, with the barrier for this second step slightly lower than the barrier for the first step. Overall, the reaction is quite exothermic.
Scheme 1 (relative energies in kcal mol-1)

|
TS1 |
3 |
|
TS2 |
4 |
Figure 1. MO6-2x/6-311+G(d) optimized Structures of 3, 4, and the transition states leading to them (TS1 and TS2).
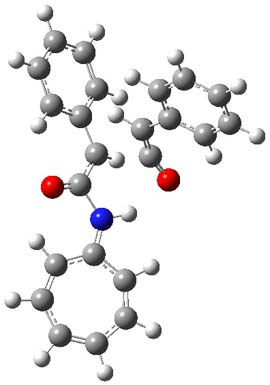
Experiments were performed with a variety of acyl chloride precursors to ketenes (Scheme 2), and along with the [8+2] product, a second product (5) incorporating 2 equivalents of ketene is found; in fact, if the R group is benzyloxy or t-butyl, 5 is the only observed product. This second product comes about via trapping of the intermediate 3. Mixing phenylketene with 4a (where the R group is phenyl) gives no reaction, thus precluding the intermediacy of 4 on the path to 5. MO6-2x computations of the trapping of 3 with phenylketenes indicates a barrier (TS3, see Figure 2) of 9.6 kcal mol-1, very close to the barrier height of the second TS for ring closure of the [8+2] pathway, supporting the competition between trapping of the intermediate and progress on to the [8+2] product.
Scheme 2.

|
TS3 |
Figure 2. MO6-2x/6-311+G(d) optimized Structures of TS3.
References
(1) Lage, M. L.; Fernandez, I.; Sierra, M. A.; Torres, M. R., "Trapping Intermediates in an [8 + 2] Cycloaddition Reaction with the Help of DFT Calculations," Org. Lett., 2011, ASAP, DOI: 10.1021/ol200910z
InChIs
1: InChI=1/C8H6O/c9-7-6-8-4-2-1-3-5-8/h1-6H
InChIKey=RZGZTQYTDRQOEY-UHFFFAOYAC
2: InChI=1/C7H7N/c8-7-5-3-1-2-4-6-7/h1-6,8H
InChIKey=NHNIVEADUHCPRP-UHFFFAOYAI
3: InChI=1/C15H13NO/c17-15(12-13-8-4-3-5-9-13)16-14-10-6-1-2-7-11-14/h1-12,17H/b15-12-/f/h17h,16H
InChIKey=AFLGFPOCUCTUAS-UJUNEOEYDT
4: InChI=1/C15H13NO/c17-15-14(11-7-3-1-4-8-11)12-9-5-2-6-10-13(12)16-15/h1-10,12,14H,(H,16,17)/t12-,14+/m1/s1/f/h16H
InChIKey=BBJCGQGFRRQHHS-KEKMKNANDW


Henry Rzepa responded on 07 Jul 2011 at 2:53 am #
To quote Yogi Berra, Deja Vu all over again! This was a hot topic in the 1970s, but then the debate was whether semi-empirical methods could handle this hot potato. I think we can say the DFT methods eventually won that one. But in 1991, I note we too were fretting on the theme of Transition state structure in cycloaddition reactions as a function of ring size and geometry, namely how many electrons could be involved in a pericyclic reaction before it turns stepwise? We decided then the transition (for a neutral hydrocarbon it has to be said) was somewhere between 10 and 14. I think that may well still be the modern view. For systems where zwitterions are more heavily stabilized, it is no surprise that it comes earlier. I do note that although the bibliography in Fernandez, Sierra and Torres article is extensive, it does not cite our article.
A related question might be whether these higher electron systems (4n+2, n=2,3) might ever sustain Möbius topologies. Thus again for a hydrocarbon (DOI: we found that two anatarafacial modes (double Möbius) could be contrived to be competitive with the purely suprafacial mechanism. Again, I suspect that if an ionic mechanism competes, it will will.
The stereochemistry of [8+2] pericyclic cycloadditions. « Henry Rzepa responded on 10 Jul 2011 at 4:17 am #
[…] Bachrach has blogged on the reaction shown below. If it were a pericyclic cycloaddition, both new bonds would form […]