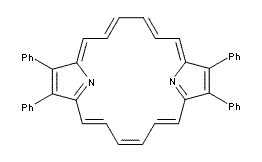
Lash has synthesized the simplified component of a porphyrin 1, which lacks two of the pyrrole rings.1 This compound should act as a modified [18]annulene, and the NMR and x-ray structure support that notion. The NMR shows a multiplet at -2.52 ppm for the internal protons and the external protons show up at 9.88 and 9.96 ppm. The x-ray structure exhibits a nearly planar structure, with the C-C distances around the macrocycle varying from 1.379 to 1.418 Å. Interestingly, the UV-vis of 1shows a Soret-band at 401 nm, indicative of porphyrin-like behavior.
|
|
|
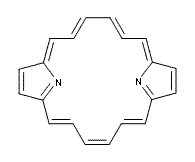
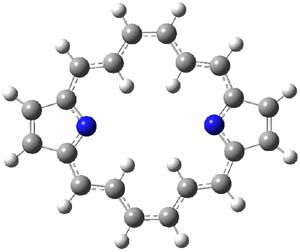
It is a simple thing to do some computations on a model of 1, and so I have computed (at B3LYP/6-311+G(d,p)) the structure and NMR of 2, shown in Figure 1. This compound is strictly planar. The C-C distances about the macrocycle vary from 1.386 to 1.415 Å, in excellent agreement with the experiment and indicating little bond alternation. The NICS values at the center of the macrocycle and 1 Å above this point are -12.6 and -11.9 ppm, supporting the aromatic [18]annulene structure. Further, the chemical shifts of the interior and exterior protons are computed to be -7.6 (interior) and 11.4 ppm (exterior) – in fair agreement with experiment. Nonetheless, simple computations provide support for the notion that this compound, and related porphyrins have a dominant [18]annulene character.
|
2 |
Figure 1. B3LYP/6-311+G(d,p) optimized structure of 2.
References
(1) Lash, T. D.; Jones, S. A.; Ferrence, G. M., "Synthesis and Characterization of Tetraphenyl-21,23-dideazaporphyrin: The Best Evidence Yet That Porphyrins Really Are the [18]Annulenes of Nature," J. Am. Chem. Soc., 2010, 132, 12786-12787, DOI: 10.1021/ja105146a
InChIs
1: InChI=1/C44H32N2/c1-2-18-30-38-42(34-23-11-6-12-24-34)44(36-27-15-8-16-28-36)40(46-38)32-20-4-3-19-3
1-39-43(35-25-13-7-14-26-35)41(37(45-39)29-17-1)33-21-9-5-10-22-33/h1-32H/b2-1-,4-3-,17-1+,18-2+,19-3+,20-4+,29-17+,30-18+,31-19+,32-20+,37-29-,38-30-,39-31-,40-32-
InChIKey=SOKXIWMGPPRDDE-VZLTUNCZBQ
2: InChI=1/C20H16N2/c1-2-6-10-18-15-16-20(22-18)12-8-4-3-7-11-19-14-13-17(21-19)9-5-1/h1-16H/b2-1-,4-3-,5-1+,6-2+,7-3+,8-4+,9-5+,10-6+,11-7+,12-8+,17-9-,18-10-,19-11-,20-12-
InChIKey=IWCJELFJBPNKQF-ICIIEBMOBH

Henry Rzepa responded on 13 May 2011 at 9:01 am #
Can any reader of this blog, or indeed its author, tell me when the first identification of a porphyrin as a Huckel 4n+2 annulene was made? I ask because the 4n+2 (more accurately the 2+4n) rule must have been circulating informally before von Doering exposed it in 1951 (DOI: 10.1021/ja01146a5370. Prior to that time, there was in fact little need for this rule, since n was almost invariably 1. Clearly, porphyrins are an example with n=4. But who was the first person to clearly state this? Please help!
Henry Rzepa responded on 13 May 2011 at 9:04 am #
Might I comment that the structures drawn above are not fully conjugated, with an implicit methylene group on the bottom lhs?
Steven Bachrach responded on 13 May 2011 at 9:22 am #
Thanks Henry for spotting this error. The figures have now been corrected.