Palau’amine has been of interest since its discovery in the early 1990s. It was just recently synthesized by Baran,1 to much acclaim. The structure of palau’amine underwent numerous revisions, and though the relative configuration had been settled, the absolute configuration was only determined by Reinscheid and Griesinger using a combination of experimental and computed ECD and ORD spectra.2
|
|
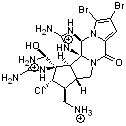
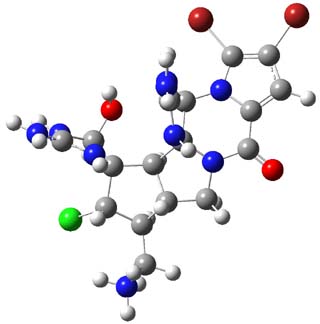
3,4-dibromopalau’amine 1 was subjected to careful NMR analysis to set as much of the overall structure as possible. Then two conformations were optimized at B3LYP/6-31G(d), one of which is displayed in Figure 1.
|
1 |
Figure 1. B3LYP/6-31G(d) optimized structure of 1.
TD-DFT computations including PCM gave an ECD spectrum that nicely matches with experiment, especially where the positive and negative peaks occur. The computed and experimental ORD spectra also match well, with all the signs matching up and a difference in the absolute value of the rotation of no more that 25%. The resulting absolute configuration is (-)-(6S,10R,11S,12S,16R,17S,18S,20S)-dibromopalau’amine, demonstrating again the power of combining computation and experiment for structure determination!
References
(1) Seiple, I. B.; Su, S.; Young, I. S.; Lewis, C. A.; Yamaguchi, J.; Baran, P. S., "Total Synthesis of Palau’amine," Angew. Chem. Int. Ed., 2010, 49, 1095-1098, DOI: 10.1002/anie.200907112
(2) Reinscheid, U. M.; Köck, M.; Cychon, C.; Schmidts, V.; Thiele, C. M.; Griesinger, C., "The Absolute Configuration of Dibromopalau’amine," Eur. J. Org. Chem., 2010, 6900-6903, DOI: 10.1002/ejoc.201001392
InChI
1: InChI=1/C17H22Br2ClN9O2/c18-6-1-7-11(30)28-3-5-4(2-21)9(20)16(13(31)25-15(23)26-16)8(5)17(28)12(24-14(22)27-17)29(7)10(6)19/h1,4-5,8-9,12-13,24-27,31H,2-3,21-23H2/q+2/p+1/t4-,5-,8+,9+,12+,13+,16+,17-/m1/s1/fC17H23Br2ClN9O2/h21H/q+3
InChIKey=VGQTUXLYZXIYTJ-CAGSSHLPDN

Henry Rzepa responded on 10 Apr 2011 at 3:16 am #
We encountered an interesting example (DOI: 10.1021/ol2001705. It concerned assignment of the absolute configuration of a helical molecule. The initial thought was to compare the ECD spectrum with a closely relatedhelicoidal molecule. It seemed to point fairly unambiguously to one configuration. But as a check, the ECD spectrum was also calculated. Despite the perceived close similarity between the two molecules, the calculated ECD reversed the configuration. The issue was resolved by measuring and calculating the ORP. In another system, the same phenomenon was again observed! We now routinely measure and calculate three chiroptical properties, ORP, ECD and VCD (with the option of a fourth, ROA) to be certain (in the absence of an anomalous dispersion X-ray structure). An article emphacizing this will (hopefully) appear quite soon.
It may be that not a few configurations assigned in the literature on the basis of suchanalogies may in fact need reversing (thus see 10.1021/ol901172g).
Tamiko Picht responded on 12 Feb 2016 at 8:27 am #
I’m more than happy to find this website. I want to to thank you for ones time due to this fantastic read!! I definitely savored every bit of it and I have you book marked to check out new things on your site.