This one is a bit afield from organic chemistry, but the result is important for computational chemists who are interested in electron density analysis.
The topological electron density analysis of Bader (also called Atoms-In-Molecules – AIM) carves up a molecular electron density into regions associated with an attractor. The attractor is a critical point in the electron density that is a maximum in all directions. Gradient paths, paths that trace increasing electron density, terminate at such an attractor. The union of all such paths defines a basin. Bader found that for typical molecules, the attractor is coincident with the position of the atomic nucleus. He has then assumed a 1:1 correspondence between these two – all nuclei are attractors and all attractors correspond with nuclei.
This correspondence has been questioned in computations on some metals. For example, Lin and Nan (n=2,4,6) have a non-nuclear attractor. However, no clear-cut unambiguous experimental observation of non-nuclear attractors has been made, until now. Platts and Stasch1 have obtained the x-ray diffraction electron density of 1 and they find a non-nuclear attractor near the midpoint of the Mg-Mg bond. This is corroborated by DFT computations of 1 and some related systems. It should be said that the electron density along the Mg-Mg path is quite flat in the middle, but the attractor is present, and the integrated number of electrons within the basin associated with this non-nuclear attractor is a non-trivial 0.81 e (experiment) or 0.79 e (DFT).
|
|
It now appears incontrovertible that non-nuclear attractors of the molecular electron density can exist. It would be especially interesting if these types of points could be located in organic species.
References
(1) Platts, J. A.; Overgaard, J.; Jones, C.; Iversen, B. B.; Stasch, A., "First Experimental Characterization of a Non-nuclear Attractor in a Dimeric Magnesium(I) Compound," J. Phys. Chem. A, 2011, 115, 194-200, DOI: 10.1021/jp109547w
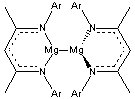

Henry Rzepa responded on 01 Mar 2011 at 1:59 pm #
A non-nuclear attractor is present in H3+, this being about the most simple system imaginable. I have also found they are not unusual in charge-shifted bonds (and metal-metal bonds are good examples of these).
Steven Bachrach responded on 01 Mar 2011 at 4:05 pm #
Is H3+ linear or a ring – and then where is the non-nuclear attractor?
I don’t believe there is a non-nuclear attractor found between the bridgehead bonds of propellane (at least not in the very old computation I did many. many years ago…) so the charge-shift bonds are not necessarily associated with this phenomenon.
Henry Rzepa responded on 03 Mar 2011 at 2:06 pm #
H3+ is a ring, and has a non-nuclear attractor at the ring centroid. It has three bond paths from each H to the non nuclear attractor. There are none between any pair of hydrogens. So in that sense, the bonds in this simple system are not like any other!
[1.1.1] propellane does not have a NNA, but it too is most unusual. They can however turn up quite unexpectedly. We have found one in quite such a situation recently!
Qadir Timerghazin responded on 29 Jun 2011 at 10:55 am #
I also note that NNAs (and thus pseudo-atoms from the AIM point of view) were observed for anionic clusters with a solvated electron, which of course makes perfect sense chemically, as we used to think of the solvated electron as a distinct chemical species.
NNAs are indeed seem to be a legitimate but rare feature in chemical bonding, but we got to be careful here – they can be just an artifact of the method!