Proton and hydrogen transfers can be catalyzed by many things. Da Silva shows that carboxylic acids can catalyze the hydrogen shift that converts an enol into a carbonyl species.1 The specific example is the ethenol to acetaldehyde tautomerization. This reaction has a barrier of 56.6 kcal mol-1 (computed using the composite method G3SX).
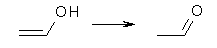
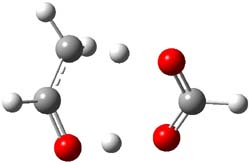
With formic acid as the catalyst, the reactant is the hydrogen-bonded complex of ethanol with formic acid and the product is the complex of acetaldehyde with formic acid. The transition state is shown in Figure 1. The barrier is only 5.6 kcal mol-1, a significant reduction. da Silva discusses how carboxylic acids might be catalyzing the enol-keto tautomerization in the troposphere and also in combustion reactions.
Figure 1. B3LYP/6-31G(2df,p) optimized TS of the formic acid catalyzed enol-keto tautomerization of acetaldehyde.
References
(1) da Silva, G., "Carboxylic Acid Catalyzed Keto-Enol Tautomerizations in the Gas Phase," Angew. Chem. Int. Ed., 2010, 49, 7523-7525, DOI: 10.1002/anie.201003530

Henry Rzepa responded on 12 Dec 2010 at 2:33 am #
Is it just me obsessed with entropy? Figure 2 in the paper referred to in this post makes it clear that the barrier of 5.6 kcal/mol is an enthalpic one, and not a free energy one. Indeed, for a bimolecular reaction, the two barriers are very different. The loss of up to six degrees of freedom in such a reaction means that a large measure of the barrier (and hence the kinetics) are due to entropy, not enthalpy. Indeed, the Eyring rate for a reaction is approximated by Ln (k/T) = 23.76 – ΔG/RT. Classical transition state theory reveals its deficiencies, since one can even have a reaction with a zero enthalpic barrier (which means formally the transition state cannot even be located on a potential energy surface), but with a finite free energy barrier. So I have to ask why articles such as this do not go the one step further and report free energy rather than enthalpy-based barriers? One might argue, as Steve has often on this blog, that a molecular dynamics based approach is probably even more realistic (and of course is the only way of handling true enthalpically barrierless reactions).
Finally, I note that the intervention of a proton-transfer agent (in this case formic acid, but more often eg water) is commonly used in many mechanisms. I note two here. Nelaine Mora-Diez has elegantly shown the process in the classical Baeyer-Villiger oxidation (DOI: 10.1039/B906058H) and I have looked at it in the ancient mechanism of aromatic electrophilic substitution as an alternative to the Wheland intermediate. There are countless others; perhaps someone should review the area?
Henry Rzepa responded on 13 Dec 2010 at 9:32 am #
Just for the record, using B3LYP/6-31G(2df,p) for energies, one gets a barrier of ~2.0 kcal/mol for the total energy barrier (not corrected for ZPE, and comparable to the 5.6 kcal/mol with, as quoted above) but 11.2 for the free energy, a somewhat different figure, but nevertheless a low value which allows the tautomerisation to occur at quite low temperatures.
Henry Rzepa responded on 23 Dec 2010 at 7:29 am #
In writing my previous comment on this post, I first had to acquire the geometry of the transition state, a Jmol model of which Steve had inlined into his post on the topic. Extracting that data is not as obvious as it might seem, and indeed in a different context, someone had castigated me for not documenting the same process in an interactive journal table. So I produced this post describing the various options on offer.
I do firmly believe that blogs such as Steve’s here, or indeed elsewhere, should be regarded as starting points for further investigations, and not just those of a couch potato inclination! The availability and re-usability of data is the key I believe to both the future of scientific journals, and other outlets such as blogs. Oh, someone told me a day or so ago that the blog was dead, long live twitter. I firmly think this is NOT true, for the very reason outline in this comment! It is also why I am worried when I hear statements like the app is the future of the web. If you want to discuss such themes, go to the spring ACS meeting in Anaheim!
Steven Bachrach responded on 23 Dec 2010 at 9:30 am #
I second Henry’s comments here in most regards. (a) Please, please include reusable structures within your publications. (b) Twitter seems to be way too limited in what can be communicated. ( c) I do believe though that the app is the future of the web – but that does not at all imply the death of the web nor necessarily any of its tools/features. I think the blog will survive. I think email survives. I think web search survives. But the specialized app on a tablet- like computer is, I think, the wave of the future.
I want to most importantly urge anyone interested in these issues to attend the session on “chemistry and the Internet” that Henry and I are organizing at the Anaheim ACS meeting. Akll of these topics and more will be discussed! I’ll post more on this session early next year.
Henry Rzepa responded on 24 Dec 2010 at 2:35 am #
Whilst we are going to have fun on some of these themes in Anaheim, I simply have to expand on the app being the future of the web. In this post I recount the story of how very difficult it has been to persuade four different commercial organisations to cooperate sufficiently to ensure that data can survive round-trips between their applications and their operating systems (specifically, the programs Chemdraw, Microsoft Office, Windows OS and Mac OS, with Acrobat thrown in for good measure). Curiously, I believe this round tripping WAS working around 1996-7, but stopped some time thereafter.
Apps (which Apple introduced I believe) are now associated with even further shrink-wrapping. It surrounds many of the tasks we used to do for ourselves (downloading files, exporting/importing into applications, etc) with the app wrapper. One simply cannot unpick these individual operations. On the iPad for example, it is the App that decides what sort of data it will render visible; no over-rides are possible! We are in the hands of the vendor as to what we can do. And, at least at the end of 2010, there is even less sign of different vendors cooperating then with the story recounted above. In 1994, we introduced chemical MIME types as a standard for allow interchange of data. With the shrink-wrapped App there is no sign of any standards for doing this emerging. And if you consider how many App stores there actually are (Apple, Android, WebOS, Microsoft, and on and on), and how there are no obvious mechanisms for data-round-tripping between different vendors apps on the horizon, I cannot feel but slightly less optimistic than Steven. But do put counter-arguments, either here or in Anaheim.
Henry Rzepa responded on 24 Dec 2010 at 10:21 am #
After I submitted the comment above, I came across this page by Jan Jensen, which is based on some innovative solutions by Kevin Theisen and ChemDoodle. This does display on e.g. an iPad. It is not (yet) an App.
It shows (some of) what might be done with the HTML5 standard (or about to be standard; I note by the way that such use of standards to draw molecules has been around a little while. Around 2002 for example, CSS and SVG were being tried for the same purpose). But I pose the same question there as I did earlier here. Can one round-trip data? Can one get data out and use it oneself? If the answer proves to be yes, then some of the confidence I lacked in the previous comment might be restored!
And a final link. To see if your device is fully up to speed on HTML5, point it at this page. I love some of the tests there; geolocation, touch, webgl, svg, etc.