Heathcock’s model for predicting the stereo-outcome of Michael additions1 involves a metal bridging across the two carbonyl oxygens. For Reaction 1, the model predicts that 1,2-syn product over the 1,2-anti product based on more favorable steric arrangements in TSA relative to TSB. Note that other rotatamers of these TS models are possible, but are presumed to be less favorable due to the inability of the metal cation to bridge the carbonyls. In fact, the syn:trans ratio for Reaction 1 is 95:5.
|
|
Reaction 1 |
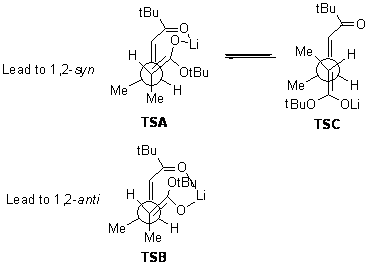
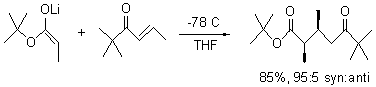
Kwan and Evans have examined this (and related) reactions at the M05-2x/6-31G(d) level.2 Dimethyl ether is used as the model for the solvent. The lowest energy transition state for Reaction 1 is TS1, shown in Figure 1 with suppressed drawing of the hydrogens (though the JMol active image will include the hydrogens). This structure is actually more like TSC, a rotamer that was thought to not have a bridging metal. TS1 does have the bridging metal, and this is accomplished by having dihedral values of 40° instead of the ideal 60°. So, computations support the general conclusion of the Heathcock approach, with a modification of the possible inclusion of some other rotamers, though the stereoprediction is not altered.
|
TS1 |
Figure 1. M05-2x/6-31G(d) optimized structure of the lowest energy transition state of Reaction 1. Hydrogens are removed in the image for clarity, but the Jmol active image (which you can see by clicking on the above image) will include the hydrogen atoms.
References
(1) Oare, D. A.; Heathcock, C. H. In Topics in Stereochemistry; Eliel, E. L., Wilen, S. H., Eds.; Wiley: New York, 1989; Vol. 19, p 227-408.
(2) Kwan, E. E.; Evans, D. A., "Intermolecular Michael Reactions: A Computational Investigation," Org. Lett. 2010, 12, 5124–5127, DOI: 10.1021/ol102017v
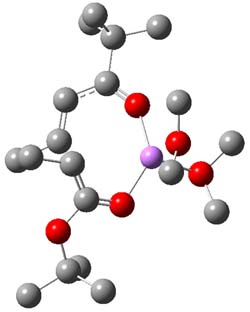

Henry Rzepa responded on 25 Nov 2010 at 1:22 am #
The lithium environment in this proposed transition state structure is conventional, in the sense of a tetrahedrally coordinated metal cation. I do note in this context that X-ray structures of lithium enolates have been determined. These are often very unusual, and depart far from the tetrahedral model of metal coordination. One such can be seen here. I have not done a thorough search for other examples, but it might be a measure of the difficulties involved in modelling Li in particular.