Monohydrated Glycolaldehyde (2-hydroxyethanal)
In Chapter 6.3.1 we discussed the conformation energy profile of solvated ethylene glycol and glycerol. The conformational preference is determined by the two competing hydrogen bonding interactions: intramolecular hydrogen bonding versus hydrogen bonding to the water molecules. For both glycerol and ethylene glycol, the lowest energy solvated structures retain the maximal number of internal hydrogen bonds possible.
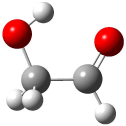
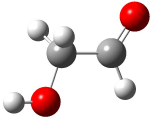
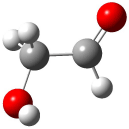
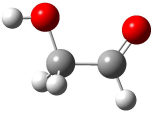
The conformational space of glycoladehyde 1 exhibits four local minima. 1 The lowest energy structure possesses an internal hydrogen bond, 1-CC (Figure 1). The other conformers are at least 3 kcal mol-1 higher in energy. Will this internal hydrogen bond persist when 1 is hydrated?
|
1-CC (0.0) |
1-TT (3.06) |
|
1-TG (3.33) |
1-CT (4.84) |
Figure 1. Optimized geometries of the conformers of 1 at MP2/aug-cc-pVTZ. 1 The letters C, T, and G indicate cis¸ trans, or gauche around the C-C and C-O bonds, in that order. Relative energies in kcal mol-1.
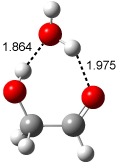
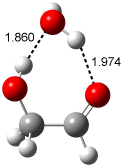
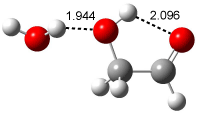
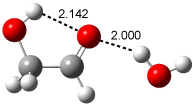
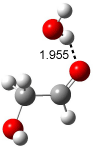
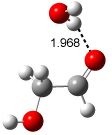
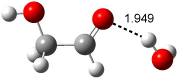
A recent computational (B3LYP/6-311++G(2df,p)) and experimental study examined the structure of monohydrated glycoladehyde.2 Optimization of the monohydrated cluster of 1 indicated that the lowest energy structures all have the glycolaldehyde fragment in the CC conformation (see Figure 2). The lowest energy cluster not in the CC arrangement is 1-TG-w, and it lies 2.86 kcal mol-1 above the lowest monohydrate, 1-CC-w1.
The lowest energy monohydrate (1-CC-w1) does not maintain the internal hydrogen bond of 1-CC. Rather, the water molecule inserts into this hydrogen bond, such that it donates a hydrogen to the carbonyl oxygen, and accepts the hydrogen from the hydroxyl group. The other conformations provide no opportunity for forming two hydrogen bonds with a water molecule, and so their hydrates are higher in energy. The microwave FT spectrum2 of the monohydrate of 1 is interpreted as a dynamic interconversion of the two lowest energy complexes, 1-CC-w1 and 1-CC-w2.
|
1-CC-w1 (0.0) |
1-CC-w2 (0.51) |
|
1-CC-w3 (0.96) |
1-CC-w4 (1.39) |
|
1-TG-w (2.86) |
1-TT-w (3.71) |
|
1-CT-w (5.98) |
Figure 2. Optimized geometries (B3LYP/6-311++G(2df,p)) of the monohydrated glycolaldehyde structures.2 All distances in Å and relative energies (G3MP2B3) in kcal mol-1.
References
(1) Ratajczyk, T.; Pecul, M.; Sadlej, J.; Helgaker, T., “Potential Energy and Spin-Spin Coupling Constants Surface of Glycolaldehyde,” J. Phys. Chem. A 2004, 108, 2758-2769, DOI: 10.1021/jp0375315
(2) Aviles-Moreno, J. R.; Demaison, J.; Huet, T. R., “Conformational Flexibility in Hydrated Sugars: the Glycolaldehyde-Water Complex,” J. Am. Chem. Soc. 2006, 128, 10467-10473, DOI: 10.1021/ja062312t