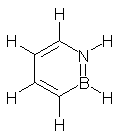
Liu has provided the link between pure the prototype organic aromatic compound (benzene) and the prototype pure inorganic aromatic (borazine).1 His group has prepared 1,2-dihydro1,2-azaborine 1. Dixon has performed computations to support the identification of the molecule. For example, the computed and experimental chemical shifts are in nice agreement (see Table 1). The B3LYP/DZVP2 optimized structure of 1 is shown in Figure 1.
|
|
Table 1. Computeda and experimental chemical shifts (ppm) of 1.1
|
|
||
|
atom |
expt |
computed |
|
B-H |
4.9 |
5.4 |
|
N-H |
8.44 |
7.8 |
|
C3–H |
6.92 |
7.3 |
|
C4–H |
7.70 |
8.0 |
|
C5–H |
6.43 |
6.6 |
|
C6–H |
7.40 |
7.4 |
|
B |
31.0 |
26.9 |
|
|
||
aB3LYP/Alhrichs-vTZP.
|
|
Figure 1. B3LYP/DZVP2 optimized structure of 1.1
The computations support the notion that 1 is truly aromatic. Its NICS(1) value is -7.27 ppm, close that of benzene (-10.39 ppm), and much more negative that that of borazine (-3.01 ppm). Reactions 1 and 2 compare the stability of 1 to benzene. These indicate that the resonance stabilization energy of 1 is about 13 kcal mol-1 less than that of benzene, whose RSE is about 34 kcal mol-1. Liu and Dixon thus consider 1 to be an aromatic compound and one that helps create a sort of organic, mixed organic-inorganic and inorganic aromatic continuum.
References
(1) Marwitz, A. J. V.; Matus, M. H.; Zakharov, L. N.; Dixon, D. D.; Liu, S.-Y., "A Hybrid Organic/Inorganic Benzene," Angew. Chem. Int. Ed. 2009, 48, 973-977, DOI: 10.1002/anie.200805554
InChIs
1: InChI=1/C4H6BN/c1-2-4-6-5-3-1/h1-6H
InChIKey=OGZZEGWWYQKMSO-UHFFFAOYAN

Computational Organic Chemistry » Aromatic stabilization energy of 1,2-azaborine responded on 15 Mar 2011 at 8:27 am #
[…] appears to be aromatic (see my previous post). Can the extent of aromatic character be measured? Well, obviously the first thing one must decide […]