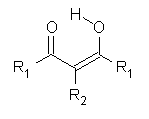
Malonaldehyde 1 possesses a very short intramolecular hydrogen bond. Its potential energy surface has two local minima (the two mirror image hydrogen-bonded structures) separated by a C2v transition state. Schaefer reports a high-level computational study for the search for even shorter hydrogen bonds that might even lead to a single well on the PES.1
|
|
||
|
1 |
R1 |
R2 |
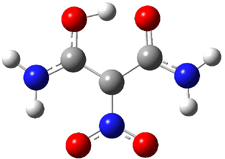
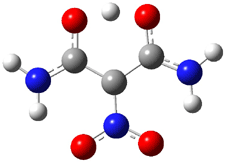
The hydrogen bond distance is characterized by the non-bonding separation between the two oxygen atoms. Table 1 shows the O…O distance for a number of substituted malonaldehydes computed at B3LYP/DZP++. Electron withdrawing groups on C2 reduce the O..O distance (see trend in 1 → 4). Electron donating groups on C1 and C3 also reduce this distance (see 5 and 6). Bulky substituents on the terminal carbons also reduce the O…O distance (see 7). Combining all of these substituent effects in 8 leads to the very short O…O distance of 2.380 Å.
Table 1. Distance (Å) between the two oxygen atoms and the barrier for hydrogen transfer of substituted malonaldehydes .1
|
Compound |
r(O…O) |
ΔEa |
ΔEb |
|
1 |
2.546 |
3.92 |
1.54 |
|
2 |
2.526 |
3.56 |
1.24 |
|
3 |
2.521 |
3.34 |
1.04 |
|
4 |
2.499 |
2.62 |
0.40 |
|
5 |
2.474 |
2.02 |
-0.06 |
|
6 |
2.498 |
|
|
|
7 |
2.466 |
|
|
|
8 |
2.380 |
0.43 |
-0.78 |
aFocal point energy. bFocal point energy and corrected for zero-point vibrational energy.
A shorter O…O distance might imply a smaller barrier for hydrogen transfer between the two oxygens. The structures of 8 and the transition state for its hydrogen transfer are shown in Figure 1. The energies of a number of substituted malonaldehydes were computed using the focal point method, and the barriers for hydrogen transfer are listed in Table 1. There is a nice correlation between the O…O distance and the barrier height. The barrier for 8 is quite small, suggesting that with some bulkier substituents, the barrier might vanish altogether, leaving only a symmetric structure. In fact, the barrier appears to vanish when zero-point vibrational energies are included.
|
8 |
8TS |
Figure 1. B3LYP/DZP++ optimized geometries of 8 and the transition state for hydrogen transfer 8TS.1
References
(1) Hargis, J. C.; Evangelista, F. A.; Ingels, J. B.; Schaefer, H. F., "Short Intramolecular Hydrogen Bonds: Derivatives of Malonaldehyde with Symmetrical Substituents," J. Am. Chem. Soc., 2008, 130, 17471-17478, DOI: 10.1021/ja8060672.
InChIs
1: InChI=1/C3H4O2/c4-2-1-3-5/h1-4H/b2-1-
InChIKey=GMSHJLUJOABYOM-UPHRSURJBI
2: InChI=1/C4H3NO2/c5-1-4(2-6)3-7/h2-3,6H/b4-2-
InChIKey=BHYIQMFSOGUTRT-RQOWECAXBC
3: InChI=1/C3H3NO4/c5-1-3(2-6)4(7)8/h1-2,5H/b3-1+
InChIKey=JBBHDCMVSJADCE-HNQUOIGGBS
4: InChI=1/C3H5BO2/c4-3(1-5)2-6/h1-2,5H,4H2/b3-1+
InChIKey=IQNKNZSFMBIPBI-HNQUOIGGBX
5: InChI=1/C3H6N2O2/c4-2(6)1-3(5)7/h1,6H,4H2,(H2,5,7)/b2-1-/f/h5H2
InChIKey=AOZIOAJNRYLOAH-KRHGAQEYDI
6: InChI=1/C5H8O4/c1-8-4(6)3-5(7)9-2/h3,6H,1-2H3/b4-3+
InChIKey=BYYYYPBUMVENKB-ONEGZZNKBI
7: InChI=1/C11H20O2/c1-10(2,3)8(12)7-9(13)11(4,5)6/h7,12H,1-6H3/b8-7-
InChIKey=SOZFXLUMSLXZFW-FPLPWBNLBX
8: InChI=1/C3H5N3O4/c4-2(7)1(3(5)8)6(9)10/h7H,4H2,(H2,5,8)/b2-1+/f/h5H2
InChIKey=IHYUFGCOUITNJP-CHFMFTGODK

Henry Rzepa responded on 15 Feb 2009 at 5:13 am #
Searches of the Cambridge crystal structure database will reveal quite a number of instances of symmetrical hydrogen bonds. These are mostly homonuclear, ie N…H…N or O…H…O. Years ago, we came across a more unusual example, of an (almost) symmetrical N….H….O heteronuclear example (http://dx.doi.org/10.1039/C39890001722 ). There are part of a general type of potential energy surface known as inverted. Apart from inverting the normal two-well X…H…H potential, there are of course also inverted C…C…C potentials, of which the norbornyl cation is a famous example. There have recently been identified inverted potential surfaces in a number of pericyclic and other types of reactions, but that deserves a separate post.
[ASK] chemistry - Do symmetric hydrogen bonds in neutral molecules exist? | Some Piece of Information responded on 05 Jun 2016 at 7:11 pm #
[…] Bachrach, 03 Feb 2009, Malonaldehydes: searching for short hydrogen bonds Also: Jacqueline C. Hargis, Francesco A. Evangelista, Justin B. Ingels and Henry F. Schaefer III, […]