It’s been a while since I blogged about the use of computed spectra to determine the structure or configuration of a compound. Well, here’s a nice example of the use of computed electronic circular dichroism to determine the configuration of a coumarin that displays axial chirality.
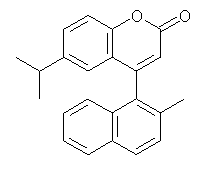
Mazzanti and coworkers have synthesized a series of coumarins,1 obtained their ECD and computed their structures, stero-interconversion barriers (at B3LYP/6-31G(d)) and ECD (at TD-DFT/B3LYP/6-311++G(2d,p)//B3LYP/6-31G(d)). I will mention explicitly here just one example, compound 1, which elutes off a chiral column in two mirror image forms, both of which do not stereomutate over time.
|
|
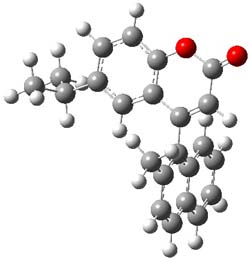
The computed structure of 1 is shown in Figure 1 and the barrier for steromutation is predicted to be quite large, 35.7 kcal mol-1. This explains the lack of stereomutation. The computed ECD of 1M matches very well with the experimental ECD of the first eluted isomer, making the second eluted isomer 1P.
|
1 |
Figure 1. B3LYP/6-31G(d) optimized structure of 1.
References
(1) Lunazzi, L.; Mancinelli, M.; Mazzanti, A.; Pierini, M., "Stereomutation of Axially Chiral Aryl Coumarins," J. Org. Chem., 2010, ASAP, DOI: 10.1021/jo101261k
InChIs
1 (6-isopropyl-4-(2-methyl-1-naphthyl)chromen-2-one):
InChI=1/C23H20O2/c1-14(2)17-10-11-21-19(12-17)20(13-22(24)25-21)23-15(3)8-9-16-6-4-5-7-18(16)23/h4-14H,1-3H3
InChIKey=OEQFRNPVUJVJFO-UHFFFAOYAS

Henry Rzepa responded on 17 Sep 2010 at 1:16 pm #
There is an interesting article on the Gaussian Web site describing V(ibrational)CD rather than E(lectronic)CD. For molecule 1, one presumes the phase of e.g. the carbonyl stretch might be quite diagnostic. Whereas ECD was introduced in an era where a priori predictions using QM were not viable, and it had to be calibrated using empirical rules, VCD is presumably of an era where computing the effect using QM was easily the most reliable (and probably only) method of calibration.