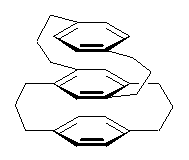
Computed optical activity was utilized in establishing the absolute configuration of the [3,3]paracyclophane 1.1 The helical twist of this molecule makes it chiral.
|
|
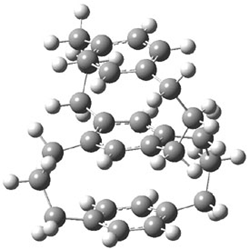
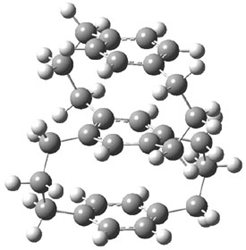
The specific rotation of (-)-1 was measured to be -123 ° [dm (g/cm3) -1]. Seven different conformations of R–1 were optimized, having either D2, C2, or C1 symmetry, at B3LYP/TZVP. The two lowest energy conformers (at B3LYP/6-31G(d) – the authors did not supply coordinates in their supporting materials!) are shown in Figure 1.
|
1a (0.0) |
1b (0.49) |
Figure 1. B3LYP/6-31G(d) optimized structures and relative energy (kcal mol-1 of the two lowest energy conformers of 1.
The TDDFT computed value for [α]D for the lowest energy conformer is -171.7 ° [dm (g/cm3)-1]. In fact, the range of [α]D for seven conformers is -124.4 to -221.8. These values are consistent with the experimental observation in both sign and magnitude. The computed CD spectrum of the seven R–1 conformations are similar to the experimental spectra of (-)-1. Thus, one can conclude that the two enantiomers are R-(-)-1 and S-(+)-1.
References
(1) Muranaka, A.; Shibahara, M.; Watanabe, M.; Matsumoto, T.; Shinmyozu, T.; Kobayashi, N., "Optical Resolution, Absolute Configuration, and Chiroptical Properties of Three-Layered [3.3]Paracyclophane(1)," J. Org. Chem., 2008, 73, 9125-9128, DOI: 10.1021/jo801441h
InChIs
1: InChI=1/C31H38/c1-3-7-28-22-30-12-6-10-27-20-18-26(19-21-27)9-5-11-29(28)23-31(30)13-4-8-25-16-14-24(2)15-17-25/h14-23H,3-13H2,1-2H3
InChIKey=GFTKQZANYFICNS-UHFFFAOYAN