The α-proton of ketones and aldehydes are acidic, thanks to delocalization of the resulting anion. However, α-protons at a bridgehead position are much less acidic – the resulting anion is not delocalized as the enolate would be an anti-Bredt alkene. So, what about more remote protons from the carbonyl – would they exhibit enhanced acidity due to inductive or field effects?
Kass has examined the deprotonation of 2-adamantone 1 via experiment and computation.1 The relative energies of the five different anions are listed in Table 1. Previous H/D exchange experiments indicate that the relative reactivity is βax > βeq > α, and this is well reproduced by computations.2
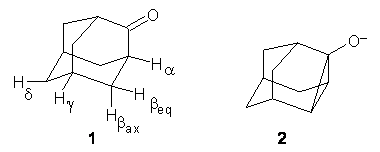
Table 1. Relative energies (kcal mol-1) of the enolates of 1.
|
|
||
|
compound |
M06-2x/aug-cc-pVDZ |
G3 |
|
|
||
|
α |
4.27 |
5.60 |
|
βax |
0.0 |
0.0 |
|
βeq |
4.46 |
|
|
γ |
2.28 |
3.40 |
|
δ |
6.17 |
7.55 |
|
2 |
-1.58 |
0.56 |
|
|
||
Kass’ bracketing experiments indicate the enthalpy for deptrotonation of 2-adamantone is 394.7 ± 1.4 kcal mol-1. This is in nice accord with the computational results for loss of the βax proton: 393.8 (M06-2x/aug-cc-pVDZ) and 396.8 kcla mol-1 (G3). One interesting computational result is a competive cyclic structure 2, whose stability is similar to that to the βax ion at M06-2x and is the optimized structure produced at MP2/6-31G(d) when searching for the βeq enolate.
So, to answer our question, protons remote from a carbonyl are more acidic than alkane
analogues, but much less acidic than typical α-protons of ketones.
References
(1) Meyer, M. M.; Kass, S. R., "Enolates in 3-D: An Experimental and Computational Study of Deprotonated 2-Adamantanone," J. Org. Chem., 2010, 75, 4274-4279, DOI: 10.1021/jo100953y
(2) Stothers, J. B.; Tan, C. T., "Adamantanone: stereochemistry of its homoenolization as shown by 2H nuclear magnetic resonance," J. Chem. Soc., Chem. Commun., 1974, 738-739, DOI: 10.1039/C39740000738
InChI
1: InChI=1/C10H14O/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-9H,1-5H2
InChIKey=IYKFYARMMIESOX-UHFFFAOYAE
2: InChI=1/C10H13O/c11-10-7-2-5-1-6(4-7)9(10)8(10)3-5/h5-9H,1-4H2/q-1
InChIKey=WTXOXRNASCZDME-UHFFFAOYAE

Henry Rzepa responded on 20 Aug 2010 at 7:11 am #
Not all bridgehead ketones are un-acidic. Here is an unusual one; A. Nickon, D. F. Covey, F.-C. Huang, and Y.-N. Kuo, Unusually facile bridgehead enolization. Locked boat forms in anti-Bredt olefins, J. Am. Chem. Soc., 1975, 97, 904 – 905; DOI:10.1021/ja00837a043. I use that example in my molecular modelling course, where the relative acidity is explained using NBO interaction energies.