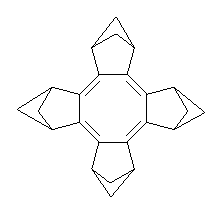
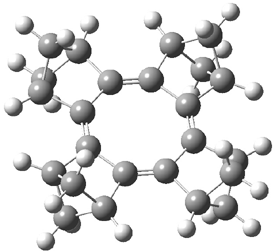
The planar substituted cyclooctatetraene 1 has been prepared and characterized.1 The B3LYP/6-31G(d) optimized geometry is shown in Figure 1.
|
|
|
|
1 |
Figure 1. B3LYP/6-31G(d) optimized geometry of 1.
The 1H NMR spectrum of 1 shows the bridgehead proton has only a small upfield shift (Δδ = 0.18ppm) relative that of 2. This suggests that both molecules have similar degrees of aromaticity/antiaromaticity, and since both molecules display large bond alternation (ΔR = 0.169 Å in 1 and 0.089 Å in 2) one can argue that both paratropic and diatropic ring currents are attenuated in both molecules. However, the NICS value of 1 is 10.6 ppm, indicative of considerable antiaromatic character, though this NICS value is much reduced from that in planar cyclooctatetraene constrained to the ring geometry of 1 (22.1 ppm). Rabinowitz and Komatsu argue that large HOMO-LUMO gap of 1 is responsible for the reduced antiaromatic character of 1.
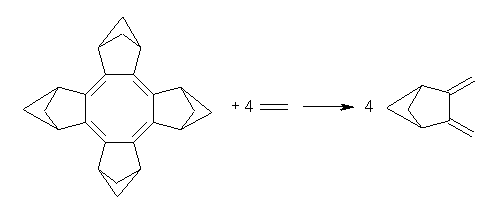
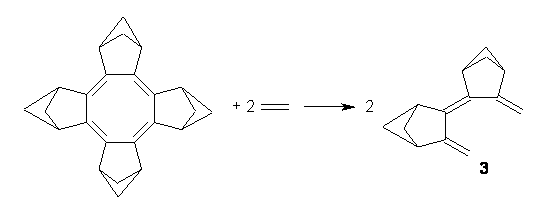
Though not discussed in their paper, the aromatic stabilization (destabilization) energy of 1 can be computed. I took two approaches, shown in Reactions 1 and 2. The energies of the two reactions are -13.8 kcal mol-1 for Reaction 1 and -3.4 kcal mol-1 for Reaction 2. The large exothermicity of Reaction 1 reflects the strain of packing the four bicyclo moieties near each other, forcing the neighboring bridgehead hydrogens to be directed right at each other. The strain is better compensated in Reaction 2 by using 3 as the reference. Since 3 is of C2 symmetry, some strain relief remains a contributor to the overall reaction energy. Thus it appears that if 1 is antiaromatic, if manifests in little energetic consequence.
Reaction 1
Reaction 2
References
(1) Nishinaga, T.; Uto, T.; Inoue, R.; Matsuura, A.; Treitel, N.; Rabinovitz, M.; Komatsu, K., "Antiaromaticity and Reactivity of a Planar Cyclooctatetraene Fully Annelated with Bicyclo[2.1.1]hexane Units," Chem. Eur. J., 2008, 14, 2067-2074, DOI: 10.1002/chem.200701405
InChIs
1: InChI=1/C24H24/c1-9-2-10(1)18-17(9)19-11-3-13(4-11)21(19)23-15-7-16(8-15)24(23)22-14-5-12(6-14)20(18)22/h9-16H,1-8H2/b19-17-,20-18-,23-21-,24-22-
InChIKey=PUZMOHQGDBIGOO-LEYBOLSUBU
2: InChI=1/C18H18/c1-7-2-8(1)14-13(7)15-9-3-11(4-9)17(15)18-12-5-10(6-12)16(14)18/h7-12H,1-6H2
InChIKey=ULLLVKXTLZQQFF-UHFFFAOYAL
3: InChI=1/C14H16/c1-7-9-3-11(4-9)13(7)14-8(2)10-5-12(14)6-10/h9-12H,1-6H2/b14-13-
InChIKey=CSIHJUFBXMYVBH-YPKPFQOOBF

Henry Rzepa responded on 15 Feb 2009 at 5:44 am #
Whilst much effort has been expended on creating a formally antiaromatic cyclo-octatetraene, another equally fascinating, and formally anti-aromatic molecule appears to have been unjustly neglected all these years. In DOI http://dx.doi.org/10.1002/anie.198801851 was reported in 1988 the x-ray structure of a [24]annulene. This also has alternating bonds (~1.36, 1.44) around its periphery. Curiously, the author of this paper (Gunther Wilke) does not mention its anti-aromaticity, which may be why the molecule has been neglected somewhat.
Indeed, in the last few years, there seems to have been something of an outbreak of antiaromatic systems, with quite a number of crystal structures now reported. The new genre of isolable antiaromatics is well and truly established.