The gas phase structure of the amino acids is in their canonical or neutral form, while their aqueous solution phase structure is zwitterionic. An interesting question is how many water molecules are needed to make the zwitterionic structure more energetically favorable than the neutral form. For glycine, it appears that seven water molecules are needed to make the zwitterion the favorable tautomer.1,2
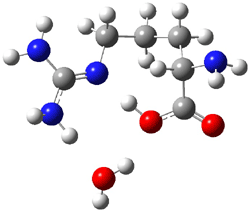
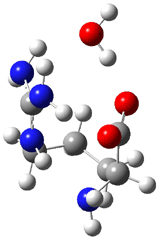
Arginine, on the other hand, appears to require only one water molecule to make the zwitterion lower in energy than the neutral form.3 The B3LYP/6-311++G** structures of the lowest energy neutral (1N) and zwitterion (1Z) cluster with one water are shown in Figure 1. The zwitterion is 1.68 kcal mol-1 lower in energy. What makes this zwitterion so favorable is that the protonation occurs on the guanidine group, not on the amine group. The guanidine group is more basic than the amine. Further, the water can accept a proton from both nitrogens of the guanidine and donate a proton to the carboxylate group.
|
1N (1.68) |
1Z (0.0) |
Figure 1. B3LYP/6-311++G** structures and relative energies (kcal mol-1) of the lowest energy arginine neutral (1N) and zwitterion (1Z) cluster with one water.3
References
(1) Aikens, C. M.; Gordon, M. S., "Incremental Solvation of Nonionized and Zwitterionic Glycine," J. Am. Chem. Soc., 2006, 128, 12835-12850, DOI: 10.1021/ja062842p.
(2) Bachrach, S. M., "Microsolvation of Glycine: A DFT Study," j. Phys. Chem. A, 2008, 112, 3722-3730, DOI: 10.1021/jp711048c.
(3) Im, S.; Jang, S.-W.; Lee, S.; Lee, Y.; Kim, B., "Arginine Zwitterion is More Stable than the Canonical Form when Solvated by a Water Molecule," J. Phys. Chem. A, 2008, 112, 9767-9770, DOI: 10.1021/jp801933y.
InChIs
1: InChI=1/C6H14N4O2/c7-4(5(11)12)2-1-3-10-6(8)9/h4H,1-3,7H2,(H,11,12)(H4,8,9,10)/f/h8,10-11H,9H2
InChIKey=ODKSFYDXXFIFQN-MYOKTFMPCK

Henry Rzepa responded on 16 Feb 2009 at 6:34 am #
The conclusion that around seven water molecules are needed to stabilize the zwitterion of glycine has been known since at least 1991, and possibly even earlier (DOI: http://dx.doi.org/10.1039/P29910000531 )
Interestingly, in order to render the SN1 solvolysis of tert-butyl chloride in water as a genuine transition state, with approximately the correct free energy of activation (and as it happens secondary deuterium isotope effects) it seems around 12-15 water molecules are needed. In both case, the waters form bridges between the positive and negative ionic centers.