Alder and co-workers have published a substantial theoretical study of potential [6+4]-cycloaddition reactions.1 There is much too much to summarize from this study, but I highlight here an interesting result that is consistent with one of the themes of the book and blog: unusual potential energy surfaces.
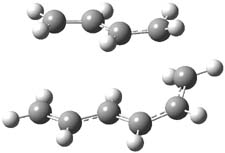
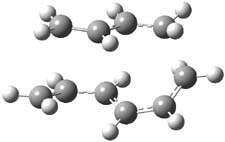
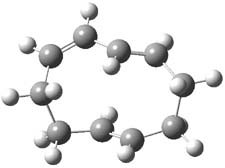
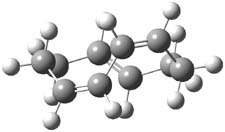
They examined two [6+4]-cycloadditon routes involving 1,3,5-hexatriene with 1,3-butadiene to give 1 and 2. These products are shown in Figure 1. A competing [4+2]-cycloaddition is also possible, giving rise to 3 and 4. Interestingly, only one TS is found leading to 1/3 and one TS leading to 2/4. (These TSs are also shown in Figure 1.) This is reminiscent of many examples from the book and blog where a single TS seems to lead to 2 different products. A valley-ridge inflection point divides the surface between 1 and 3 (VRI-1), and a second valley-ridge inflection point separates 2 from 4 (VRI-2). In addition a Cope transition state (CTS1) takes 1 into 3, and a second TS (CTS2) takes 2 into 4.

|
TS1 |
TS2 |
|
1 |
2 |
|
CTS1 |
CTS2 |
Figure 1. B3LYP/6-31G* optimized structures of the TSs and products of the reaction of 1,3,5-hexadiene with 1,3-butadiene.1
This type of surface requires study of the dynamics to truly predict what the outcome will be of the reaction. Unfortunately, the low barriers for the Cope rearrangements along with 3 and 4 being much more stable than 1 and 2 indicates that the [6+4] product is unlikely to be observed. Nonetheless, this is yet another example of an unexpected PES.
References
(1) Alder, R. W.; Harvey, J. N.; Lloyd-Jones, G. C.; Oliva, J. M., "Can π6 + π4 = 10? Exploring Cycloaddition Routes to Highly Unsaturated 10-Membered Rings," J. Am. Chem. Soc. 2010, 132, 8325-8337, DOI: 10.1021/ja1008135
InChIs
1: InChI=1/C10H14/c1-2-4-6-8-10-9-7-5-3-1/h1-4,9-10H,5-8H2/b3-1-,4-2+,10-9+
InChIKey=RBGHZLIWLPEVLM-OCXPBMDHBA
2: InChI=1/C10H14/c1-2-4-6-8-10-9-7-5-3-1/h1-4,9-10H,5-8H2/b3-1-,4-2-,10-9+
InChIKey=RBGHZLIWLPEVLM-ARMDLRMMBD
3: InChI=1/C10H14/c1-3-9-7-5-6-8-10(9)4-2/h3-5,7,9-10H,1-2,6,8H2/t9-,10-/m0/s1
InChIKey=ANOQDGNLTWJTRB-UWVGGRQHBI
4: InChI=1/C10H14/c1-3-9-7-5-6-8-10(9)4-2/h3-5,7,9-10H,1-2,6,8H2/t9-,10+/m1/s1
InChIKey=ANOQDGNLTWJTRB-ZJUUUORDBZ
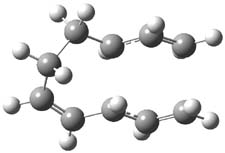
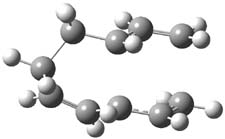

Henry Rzepa responded on 21 Jul 2010 at 6:38 am #
An interesting challenge would be to subvert this reaction into a π6a + π4a mode, i.e. with two antarafacial rather than two suprafacial components. This mode does not seem to have been explored in the article Steve cites. I suspect though that it might be a challenge to produce substituents that block all the more simple routes in a steric sense, leaving only this mode. It would nevertheless be of interest as an example of what might be called a double-twist Möbius system. Although examples of such topologies are well known for hexa and octa-phyrins, they are as yet unknown for transition states. The transition state for this one can be inspected here, where it has formed a component of my taught course on pericyclic reactions for a few years now.