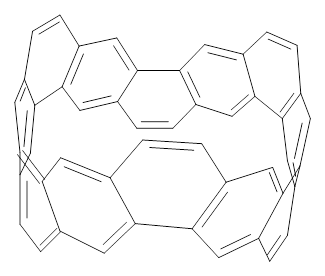
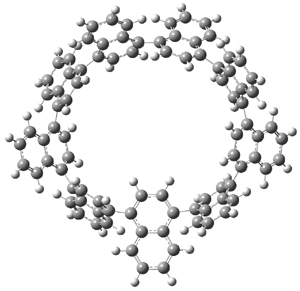
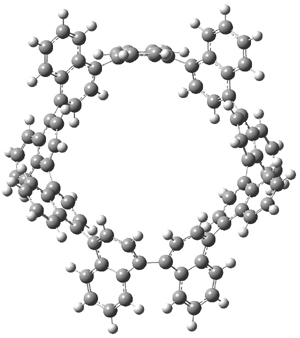
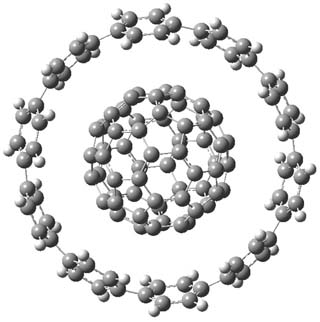
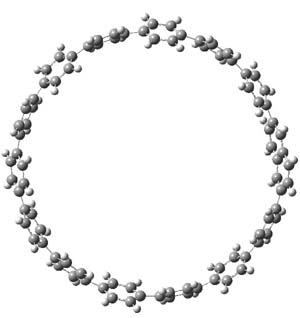
The synthesis of components of nanostructures (like fullerenes and nanotubes) has dramatically matured over the past few years. I have blogged about nanohoops before, and this post presents the recent work of the Itami group in preparing the nanobelt 1.1
|
|
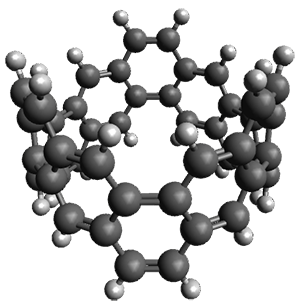
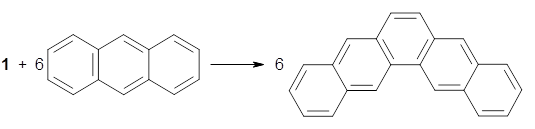
The synthesis is accomplished through a series of Wittig reactions with an aryl-aryl coupling to stitch together the final rings. The molecule is characterized by NMR and x-ray crystallography. The authors have also computed the structure of 1 at B3LYP/6-31G(d), shown in Figure 1. The computed C-C distances match up very well with the experimental distances. The strain energy of 1, presumably estimated by Reaction 1,2 is computed to be about 119 kcal mol-1.
|
1 |
Figure 1. B3LYP/6-31G(d) optimized structure of 1.
|
|
Rxn 1 |
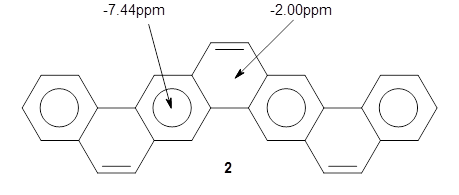
NICS(0) values were obtained at B3LYP/6-311+G(2d,p)//B3LYP/6-31G(d); the rings along the middle of the belt have values of -7.44ppm and are indicative of normal aromatic 6-member rings, while the other rings have values of -2.00ppm. This suggests the dominant resonance structure shown below:
References
1) Povie, G.; Segawa, Y.; Nishihara, T.; Miyauchi, Y.; Itami, K., "Synthesis of a carbon nanobelt." Science 2017, 356, 172-175, DOI: 10.1126/science.aam8158.
2) Segawa, Y.; Yagi, A.; Ito, H.; Itami, K., "A Theoretical Study on the Strain Energy of Carbon Nanobelts." Org. Letters 2016, 18, 1430-1433, DOI: 10.1021/acs.orglett.6b00365.
InChIs:
1: InChI=1S/C48H24/c1-2-26-14-40-28-5-6-31-20-44-32(19-42(31)40)9-10-34-24-48-36(23-46(34)44)12-11-35-21-45-33(22-47(35)48)8-7-30-17-41-29(18-43(30)45)4-3-27-15-37(39(26)16-28)25(1)13-38(27)41/h1-24H
InChIKey=KJWRWEMHJRCQKK-UHFFFAOYSA-N