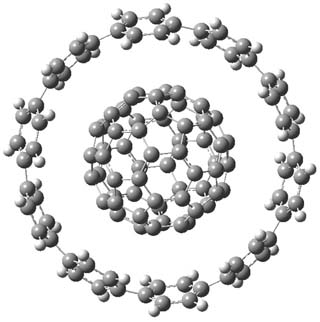
Check out this an incredibly cool host guest complex: the [10]-cycloparaphenylene ([10]CPP) hoop encapsulating C60!1
(Be sure to click on this image to bring up the 3-D interactive structure – as with all structures in my blog!)
1H and 13C NMR and fluorescence quenching spectrometry clearly indicate that this complex is formed when [10]CPP is mixed with C60 in toluene. In fact, when C60 is mixed with a mixture of nanohoops ranging from 8 to 12 phenyl ring, only the [10]CPP hoop complexes with the fullerene. The experimental binding energy is between 38 and 59 kJ mol-1.
M06-2x/6-31G* computations give the structure shown above. The computed binding energy is 173 kJ mol-1, but the computations do not include solvent. So this overestimation might be somewhat due to the difference in gas phase vs. solution complexation.
(Check out this post for other interesting nanohoops.)
References
(1) Iwamoto, T.; Watanabe, Y.; Sadahiro, T.; Haino, T.; Yamago, S., "Size-Selective Encapsulation of C60 by [10]Cycloparaphenylene: Formation of the Shortest Fullerene-Peapod," Angew. Chem. Int. Ed., 2011, 50, 8342-8344, DOI: 10.1002/anie.201102302

Henry Rzepa responded on 18 Sep 2011 at 4:47 am #
As you say Steve, a lovely molecule.
Can I clarify one aspect. The article mentions that [10]CPP and C60 are stabilised by about 38 kJ. This seems to come from binding constant measurements, and so we may take it to be a Gibbs free energy. Likewise, the activation energy for the exchange reaction is a free energy obtained by the coalescence temperature in the NMR spectra. However, the calculation reported claims to be an enthalpy (actually, its not even that, since I think it does not include any zero-point energy thermal corrections). Since we are dealing with loss of degrees of freedom resulting from encapsulation, enthalpies and free energies must be related by an evaluated entropy.
I notice that many an article reports energies whilst being rather vague about whether they are enthalpies, free energy, binding or activation energies (Eyring or otherwise).
Molecular Matryoshka dolls « Henry Rzepa responded on 20 Sep 2011 at 9:08 am #
[…] nesting doll. They can have up to eight layers. Molecules can only emulate two layers, although see here for a good candidate for making a three-layered example (the inside layer is C60, which itself […]
Computational Organic Chemistry » nano-Saturn responded on 28 Aug 2018 at 10:53 am #
[…] It never hurts to promote one’s science through clever names – think cubane, buckminsterfullerene, bullvalene, etc. Host-guest chemistry is not immune to this cliché too, and this post discusses the latest synthesis and computations of a nano-Saturn; nano-Saturns are a spherical guest molecule captured inside a ring host molecule. I discussed an example of this a number of years ago – the nano-Saturn comprised of C60 fullerene surrounded by [10]cycloparaphenylene. […]