The competition between Bergman cyclization and Myers-Saito cyclization of ene-ynes and related species is discussed in Chapter 3.3 of my book and also in these posts. Yet another variation, the Garratt-Braverman cyclization1-3 has now been examined in terms of competition with the Myers-Saito cyclization for 1 using both experiments and computations.4 Subjecting 1 to base should cause the rearrangement to either GB1 or MS2. These can undergo either the Garratt-Braverman cyclization to give GB2 or the Myers-Saito cyclization to MS2.

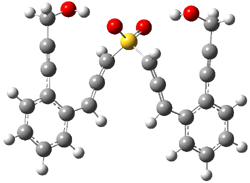
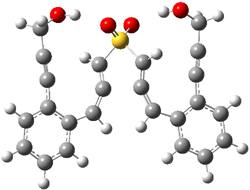
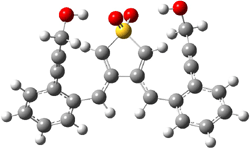
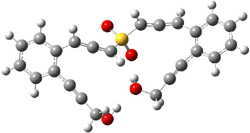
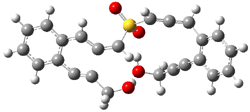
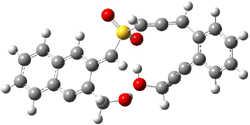
B3LYP/6-31G(d) predicts that GB1 is only slightly higher in energy than MS1 (by 0.7 kcal mol-1). The transition states (GB1toGB2 or MS1toMS2 – see Figure 1) each lie 24.4 kcal mol-1 above their respective reactants. However, the diradical GB2 is 7.2 kcal mol-1 below GB1 but MS2 is only 0.3 kcal mol-1 below MS1. So while the two reactions are of similar kinetic probability, having identical activation barriers, the GB route leads to the more thermodynamically stable intermediate. Furthermore, the GB route ultimately results in GBP, via an intramolecular cyclization of the diradical, while the MS route, which ends with MSP, requires intermolecular abstraction of 4 hydrogens. Thus, the unimolecularity of the GB path further favors the GB route over the MS pathway. In fact, experimental studies of 1 and related compounds all give rise to the GB product only.
|
GB1 |
GB1toGB2 |
|
GB2 |
|
|
|
MS1toMS2 |
|
MS2 |
|
Figure 1. B3LYP/6-31G(d) optimized structures.4
References
(1) Braverman, S.; Segev, D., "Novel cyclization of diallenic sulfones," J. Am. Chem. Soc. 2002, 96, 1245-1247, DOI: 10.1021/ja00811a060
(2) Garratt, P. J.; Neoh, S. B., "Strained heterocycles. Properties of five-membered heterocycles fused to four-, six-, and eight-membered rings prepared by base-catalyzed rearrangement of 4-heterohepta-1,6-diynes," J. Org. Chem. 2002, 44, 2667-2674, DOI: 10.1021/jo01329a016
(3) Zafrani, Y.; Gottlieb, H. E.; Sprecher, M.; Braverman, S., "Sequential Intermediates in the Base-Catalyzed Conversion of Bis(π-conjugated propargyl) Sulfones to 1,3-Dihydrobenzo- and Naphtho[c]thiophene-2,2-dioxides," J. Org. Chem. 2005, 70, 10166-10168, DOI: 10.1021/jo051692i
(4) Basak, A.; Das, S.; Mallick, D.; Jemmis, E. D., "Which One Is Preferred: Myers-Saito Cyclization of Ene-Yne-Allene or Garratt-Braverman Cyclization of Conjugated Bisallenic Sulfone? A Theoretical and Experimental Study," J. Am. Chem. Soc. 2009, 131, 15695-15704, DOI: 10.1021/ja9023644