I recently posted on a paper proposing 1,2-benzoquinone and related compounds as the diene component for bioorthogonal labeling. Levandowski, Gamache, Murphy, and Houk report on tetrachlorocyclopentadiene ketal 1 as an active ambiphilic diene component.1
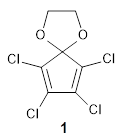
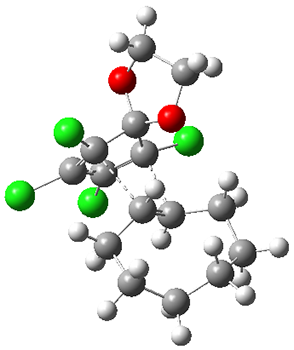
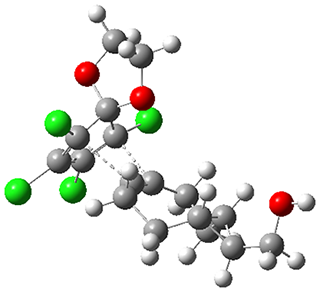
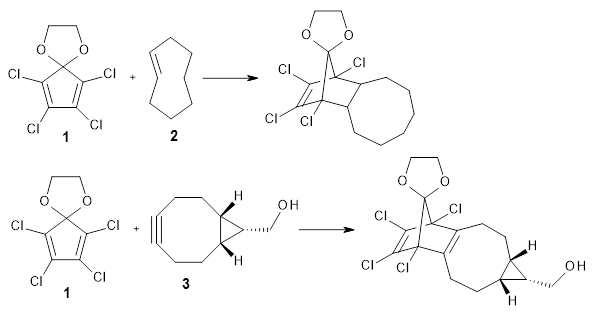
1 is sterically congested to diminish self-dimerization and will react with both electron-rich and electron-poor dienes. To test it as an active diene in bioorthogonal labeling applications, they optimized the structures of the transition states at CPCM(water)/M06-2X/6-311+G(d,p)//CPCM(water)/M06-2X/6-31G(d) for the Diels-Alder reaction of 1 with a variety of dienophiles including trans-cyclooctene 2 and endo-bicyclononyne 3. These transition states are shown in Figure 1. The activation free energy is quite low for each: 18.1 kcal mol-1 with 2 and 18.9 kcal mol-1 with 3.
|
|
|
Figure 1. CPCM(water)/M06-2X/6-31G(d) optimized geometries for the TSs of the reaction of 1 with 2 and 3.
Experiments were successfully run using 1 as a label on a neuropeptide.
References
1) Levandowski, B. J.; Gamache, R. F.; Murphy, J. M.; Houk, K. N., "Readily Accessible Ambiphilic Cyclopentadienes for Bioorthogonal Labeling." J. Am. Chem. Soc. 2018, 140, 6426-6431, DOI: 10.1021/jacs.8b02978.
InChIs
1:InChI=1S/C7H4Cl4O2/c8-3-4(9)6(11)7(5(3)10)12-1-2-13-7/h1-2H2
InChIkey=DXQQKKGWMVTLOJ-UHFFFAOYSA-N