Here’s another cruel and unusual punishment applied to the poor benzene ring. Hashimoto,et al. have created a molecule that is a fused double helicene, where the fusion is about a single phenyl ring.1 Compound 1 has two [5]helicenes oriented in opposite directions. This should provide a twist to the central phenyl ring, and the added methyl groups help to expand that twist.
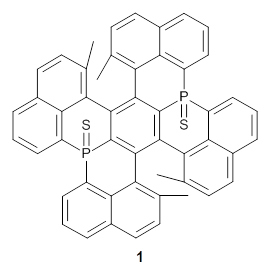
They prepared 1 and its x-ray crystal structure is reported. The compound exhibits C2 symmetry. The twist (defined as the dihedral of four consecutive carbon atoms of the central ring) is 28.17°, nearly the same twist as in [2]paraphenylene.
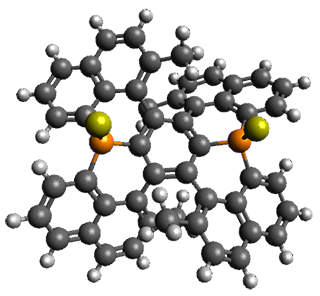
The B3LYP/6-31G(d) structure of 1 is shown in Figure 1. This geometry is very similar to the x-ray structure. The calculated NICS value for the central ring is -4.9 (B3LYP/6-311+G(d,p)/B3LYP/6-31G(d)) and -4.3 (B3LYP/6-311+G(d,p)/x-ray structure). This diminished value from either benzene or C6(PSH2)2(CH3)4 indicates reduced aromaticity of this central ring, presumably due to the distortion away from planarity. Nonetheless, the central ring of 1 is not oxidized when subjected to MCPBA to oxidize to the bis phosphine oxides.
|
1 |
Figure 1. B3LYP/6-31G(d) optimized structure of 1.
References
(1) Hashimoto, S.; Nakatsuka, S.; Nakamura, M.; Hatakeyama, T. "Construction of a Highly Distorted Benzene Ring in a Double Helicene," Angew. Chem. Int. Ed. 2014, 53, 14074-14076, DOI: 10.1002/anie.201408390.
InChIs
1: InChI=1S/C50H32P2S2/c1-25-17-21-29-9-5-13-33-41(29)37(25)45-46-38-26(2)18-22-30-10-7-15-35(42(30)38)52(54)36-16-8-12-32-24-20-28(4)40(44(32)36)48(50(46)52)47-39-27(3)19-23-31-11-6-14-34(43(31)39)51(33,53)49(45)47/h5-24H,1-4H3