PETN C(CH2ONO2)3 is a relatively insensitive explosive. The silicon analogue Si(CH2ONO2)3 is extraordinarily sensitive, exploding at the touch of a spatula. (By the way, this makes it extremely ill-advised as an explosive – it’s way too dangerous!) Goddard employed MO6 computations to explore five different possible decomposition pathways, shown in Scheme 1.1 Reaction 1, the loss of NO2, is a standard decomposition pathway for many explosives, but the barrier for the C and Si analogues are similar and the reaction of the Si compound is not exothermic. The barrier for Reaction 2 is very large, and the barriers for the C and Si analogues for Reactions 3 and 4 are too similar to explain the differences in their sensitivities.
Scheme 1.
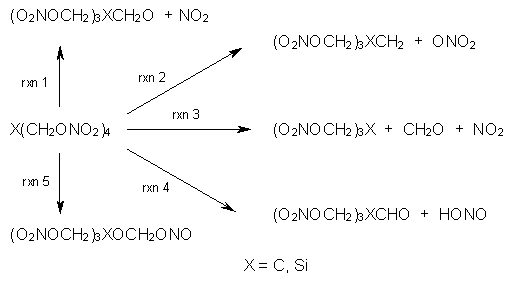
Reaction 5, however, does offer an explanation. The barrier for the Si analogue is 32 kcal mol-1, lower than for any other pathway, and almost 50 kcal mol-1 lower than the barrier for the rearrangement of the PETN itself. Furthermore, Reaction 5 is very exothermic for Si-PETN (-44.5 kcal mol-1), while the most favorable pathway for PETN decomposition, Reaction 1, is endothermic. Thus the small barrier and the large amount of energy released for Reaction 5 of Si-PETN suggests its extreme sensitivity.
References
(1) Liu, W.-G.; Zybin, S. V.; Dasgupta, S.; Klapötke, T. M.; Goddard III, W. A., "Explanation of the Colossal Detonation Sensitivity of Silicon Pentaerythritol Tetranitrate (Si-PETN) Explosive," J. Am. Chem. Soc. 2009, 131, 7490-7491, DOI: 10.1021/ja809725p.
InChIs
PETN: InChI=1/C5H8N4O12/c10-6(11)18-1-5(2-19-7(12)13,3-20-8(14)15)4-21-9(16)17/h1-4H2
InChIKey=TZRXHJWUDPFEEY-UHFFFAOYAE
Si-PETN: InChI=1/C4H8N4O12Si/c9-5(10)17-1-21(2-18-6(11)12,3-19-7(13)14)4-20-8(15)16/h1-4H2
InChIKey=FBKTZZKPJKPXMT-UHFFFAOYAL

Steve L responded on 14 Sep 2011 at 12:06 pm #
PETN is actually very sensitivity. So sensitive in fact it is able to be initiated by 1 mg lead azide. Check the encyclopedia of explosive produced by the Picatinney Arsenal.