Kemnitz and co-workers have added to the protobranching debate (see these earlier posts i, ii, iii) with a proposal for how branching can be stabilizing.1 A normal chemical bond can be described within the valence bond prescription as an interplay of three different contributors: a covalent term (a) and two ionic terms (b and c). For a typical covalent bond, term a dominates, and for the recently proposed “charge-shift” bond (see this post), the ionic VB terms dominate.
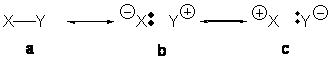
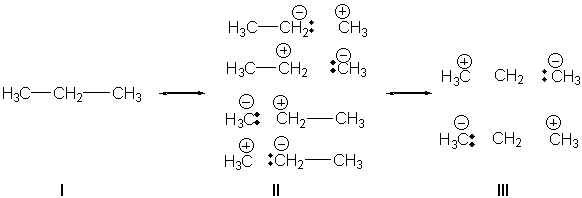
Kemnitz now examines propane using a valence bond method and finds the following. The dominant VB term is the standard, two-covalent bond structure I. Next in importance are the single bond ionic VB structures II. Lastly, the 1,3-ionic structures III contribute about 9% to the total VB wavefunction. These contributions are only possible with branching and provide a net stabilization of about 1.6 kcal mol-1. This energy is nearly identical to the stabilization energy associated with the protobranching concept proposed by Schleyer, Houk and Mo. This type of ionic structure just might be the mechanism for protobranching stabilization.
References
(1) Kemnitz, C. R.; Mackey, J. L.; Loewen, M. J.; Hargrove, J. L.; Lewis, J. L.; Hawkins, W.
E.; Nielsen, A. F., "Origin of Stability in Branched Alkanes," Chem. Eur. J. 2010, 16,6942-6949, DOI: 10.1002/chem.200902550
