Following up on his previous studies of isotope effects on the ring opening of cyclopropylcarbinyl radical 1 to give 2 (see my previous post), Borden now reports on its kinetic isotope effect (KIE).1
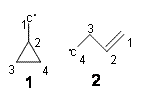
Using the small-curvature tunneling approximation along with structures and frequencies computed at B3LYP/6-31G(d), he finds a negligible KIE at C1, consistent with little motion of C1 in the transition vector. The KIE for substitution at C4 is large (k(12C/14C)=5.46), also consistent with its large motion in the transition vector. What is surprising is the KIE for deuterium substitution at C1: 0.37. This is a large inverse isotope effect!
Analysis of the vibrational frequencies that involve the C1 hydrogens provides an explanation. In going to the TS for the ring opening, both the torsional motion about the C1-C2 bond (making the double bond) and the pyramidal motion increase in frequency. This leads to a higher activation barrier for H than D, and the inverse isotope effect.
References
(1) Zhang, X.; Datta, A.; Hrovat, D. A.; Borden, W. T., “Calculations Predict a Large Inverse H/D Kinetic Isotope Effect on the Rate of Tunneling in the Ring Opening of Cyclopropylcarbinyl Radical,” J. Am. Chem. Soc., 2009, 131, 16002-16003, DOI: 10.1021/ja907406q.

Henry Rzepa responded on 06 Jan 2010 at 1:37 am #
You note that the surprising result from Borden’s work is the inverse H/D isotope effect, but I find more surprising the quoted reported magnitude of the 12C/14C effect (k(14C/12C)=5.46 in the blog). I have checked Borden’s article, and the magnitude is indeed what is quoted (although its not discussed in the text unless I missed it). In my experience, carbon isotope effects are normally measured as small percentages, and I think it far more likely that what was meant was a 5.46% effect, ie 1.055. A 546% effect, which is how Borden’s paper reads, would be way by far the largest carbon isotope effect ever recorded (and since carbon does not really tunnel, it cannot be due to that effect), and indeed is up there with hydrogen isotope effects!
There is a minor error in the blog, it should be k(12C/14C) rather than the way you have it (heavier isotopes do normally react more slowly).
Have I missed something in Borden’s article, or should we invite the man himself to comment on this blog?
Steven Bachrach responded on 06 Jan 2010 at 9:11 am #
Thanks Henry for catching the error in the blog – I have corrected the ratio.
I agree that the carbon isotope effect is likely to be interpreted at 5.46% – but I shall send Wes an email for clarification.
Wes responded on 06 Jan 2010 at 3:48 pm #
Dear Steve and Henry,
The KIE of 5.46 is correct. Please remember that this is a KIE on tunneling by an atom that moves a lot in the ring opening of cyclopropylcarbinyl radical, and 12C → 14C increases its mass by 17% (and the mass of the entire CH2 group by 14%). As you know, tunneling is very sensitive to mass, which is why heavy-atom tunneling is comparatively rare.
With best wishes to both of you for the New Year,
Wes