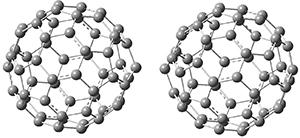
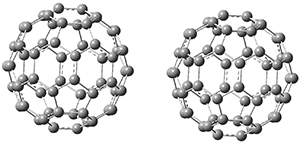
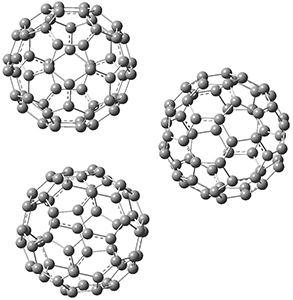
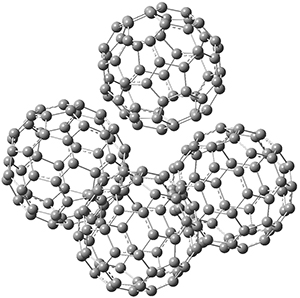
Clark and co-workers have examined small fullerene clusters for their ability to capture electrons.1 They first looked at the fullerene dimer, comparing the electron affinity of the dimer having a C-C bond between the two cages (about 1.6-1.7 Å between the two cages) 1 and where the two cages are interacting only through van der Waals attractions (around 2.6 Å) 2. The structures and their radical anions were computed at RI-BP86/TZV. The structures of the two radical anions are shown in Figure 1. Interestingly, the radical anion of 2 is actually lower in energy that the radical anion of 1. Comparisons with some other methods are discussed, including a CASSPT2(5,4)/ANO-L-VDZ, computation, that support this result.
|
1 |
2 |
|
3 |
4 |
Figure 1. RI-BP86/TZV optimized geometries of the radical anions of 1-4.
(Be sure to click on these images to be able to manipulate these structures in 3-D!)
This suggests that the added electron is being held between the cages, in an interstitial region. That suggested looking at the trimer and tetramer structures 3 and 4. The radical anions of these two oligomers are also shown in Figure 1. These oligomers show electron affinities of 1 eV greater than for fullerene itself, along with the ability to stabilize the dianion and even the trianion, what the authors call “deep electron traps”.
References
(1) Shubina, T. E.; Sharapa, D. I.; Schubert, C.; Zahn, D.; Halik, M.; Keller, P. A.; Pyne, S. G.; Jennepalli, S.; Guldi, D. M.; Clark, T. "Fullerene Van der Waals Oligomers as Electron Traps," J. Am. Chem. Soc. 2014, 136, 10890-10893, DOI: 10.1021/ja505949m.